Chemistry:Muironolide A
| |
| Names | |
|---|---|
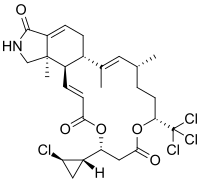
| IUPAC name
(1S,2E,4R,7R,11S,14E,16R,17R)-11-[(1S,2R)-2-chlorocyclopropyl]-2,4,17-trimethyl-7-(trichloromethyl)-8,12-dioxa-19-azatricyclo[14.7.0.017,21]tricosa-2,14,21-triene-9,13,20-trione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H33Cl4NO5 | |
| Molar mass | 593.36 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Muironolide A is a tetrachloro polyketide discovered in 200 that has two unusual structural details: a hexahydro-1H-isoindolone-triketide ring and a trichlorocarbinol ester. It is suspected that it is the product of a sponge–microorganism (cyanobacteria) association.[1] It was isolated from the marine sponge Phorbas sp.
Biosynthesis and synthesis
Muironolide A possibly has its biosynthetic route coming from PKS 1 responsible for forming the lactonic ring, with an amino acid residue, which forms the isoindole ring present in the molecule and successive enzymatic transformations of reduction, oxidation, cyclication, denaturation and additions of halogens ( Chlorine - Cl) result in the final molecule.[2]
There are proposals for synthetic routes that elucidate the synthesis process. In 2015, Xiao and collaborators carried out the synthesis and structural revision of muironolide A molecules.[2]
Biological activities
Muironolide A has already been tested for antineoplastic activity in 56 different models using different cell lines and did not provide biological activity in any of them.[1] Phorbas sp. also produces the macrolides phorboxazoles A and B and phorbaside A, which do have antifungal and cytostatic activity. [1]
References
- ↑ Jump up to: 1.0 1.1 1.2 Dalisay, Doralyn S.; Morinaka, Brandon I.; Skepper†, Colin K.; Molinski, Tadeusz F. (May 15, 2009). "A Tetrachloro Polyketide Hexahydro-1H-isoindolone, Muironolide A, from the Marine Sponge Phorbas sp. Natural Products at the Nanomole Scale". Journal of the American Chemical Society 131 (22): 7552–7553. doi:10.1021/ja9024929. PMID 19453148.
- ↑ Jump up to: 2.0 2.1 Xiao, Qing; Young, Kyle; Zakarian, Armen (2015-05-04). "Total Synthesis and Structural Revision of (+)-Muironolide A". Journal of the American Chemical Society 137 (18): 5907–5910. doi:10.1021/jacs.5b03531. ISSN 0002-7863. PMID 25928351. http://dx.doi.org/10.1021/jacs.5b03531.
 |

