Chemistry:N-Methylmethanimine
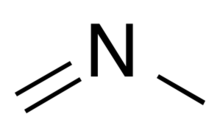
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H5N | |
| Molar mass | 43.069 g·mol−1 |
| Related compounds | |
Related compounds
|
dimethylamine Methanimine Ethanimine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Methylmethanimine or N‐methyl methylenimine is a reactive molecular substance containing a methyl group attached to an imine. It can be written as CH3N=CH2. On a timescale of minutes it self reacts to form a trimer, of trimethyl 1,3,5-triazinane. N-Methylmethanimine is formed naturally in the Earth's atmosphere, by oxidation of dimethylamine and trimethylamine, both of which are produced by animals, or burning.[1]
Production
N-Methylmethanimine can be produced in two steps from dimethylamine, by first chlorinating the nitrogen atom with solid N-chlorosuccinimide, and then treating with potassium tert-butoxide at 90°C.[2] Also it can be formed directly by thermal decomposition.[3]
It can also be prepared from the trimer: 1,3,5-trimethyl-1,3,5-triazinane by heating to 450 °C (842 °F).[2]
At 515 °C (959 °F), trimethylamine decomposes into methane and N-methylmethanimine.[4]
Natural occurrence
N-Methylmethanimine should be formed in the atmosphere as a result of degradation by oxidation of di- and trimethylamine. These occur at concentrations of a few parts per billion. But N-methylmethanimine cannot be detected. This is likely because it forms the trimer, gets absorbed onto particles, such as cloud droplets, and hydrolyses to form methylamine and formaldehyde.[1]
Properties
The N-Methylmethanimine molecule has Cs symmetry.[2] The infrared spectrum and microwave spectrum have been observed.[5]
The bond length for C=N is 1.279 Å, and for the N-C bond it is 1.458 Å. The ∠ C=N-C is 116.6°.[4]
The electric dipole moment is 1.53 Debye.[6]
When heated to 535°, N-methylmethanimine decomposes to hydrogen cyanide (HCN) and methane (CH4).[4]
Between 400 and 550°C, the cyclic amine, aziridine decomposes to a mixture of N-methylmethanimine and ethylideneimine.[7]
References
- ↑ 1.0 1.1 Bunkan, Arne Joakim C.; Reijrink, Nina G.; Mikoviny, Tomáš; Müller, Markus; Nielsen, Claus J.; Zhu, Liang; Wisthaler, Armin (11 May 2022). "Atmospheric Chemistry of N -Methylmethanimine (CH 3 N═CH 2 ): A Theoretical and Experimental Study". The Journal of Physical Chemistry A 126 (20): 3247–3264. doi:10.1021/acs.jpca.2c01925. PMID 35544412.
- ↑ 2.0 2.1 2.2 Demaison, J.; Burie, J.; Denis, J.M.; Van Eijck, B.P. (October 1984). "Centrifugal distortion and internal rotation analysis of the rotational spectra of N-methylmethanimine d0 and d5". Journal of Molecular Spectroscopy 107 (2): 250–260. doi:10.1016/0022-2852(84)90004-3.
- ↑ Mulcahy, Christopher P.A.; Carman, April J.; Casey, Sean M. (July 2000). "The adsorption and thermal decomposition of dimethylamine on Si(100)". Surface Science 459 (1–2): 1–13. doi:10.1016/S0039-6028(00)00487-8.
- ↑ 4.0 4.1 4.2 Fujiwara, Hideo; Egawa, Toru; Konaka, Shigehiro (January 1995). "Electron diffraction study of thermal decomposition products of trimethylamine: molecular structure of CH3NCH2". Journal of Molecular Structure 344 (3): 217–226. doi:10.1016/0022-2860(94)08440-S.
- ↑ Yu, Gang; Duxbury, Geoffrey (September 1992). "High-resolution diode laser spectroscopy of the ν16 band of N-methylmethylenimine". Journal of Molecular Spectroscopy 155 (1): 105–114. doi:10.1016/0022-2852(92)90551-X.
- ↑ Sastry, K. V. L. N.; Curl Jr., R. F. (1964). "Microwave Spectrum of N‐Methyl Methylenimine". J. Chem. Phys. 41: 77. doi:10.1063/1.1725653.
- ↑ Stolkin, I.; Ha, T.-K.; Günthard, Hs.H. (May 1977). "N-methylmethyleneimine and ethylideneimine: Gas- and matrix-infrared spectra, AB initio calculations and thermodynamic properties". Chemical Physics 21 (3): 327–347. doi:10.1016/0301-0104(77)85189-6.
 |

