Chemistry:Nargenicin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Excretion | ~ |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
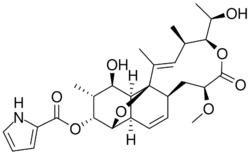
| Formula | C28H37NO8 |
| Molar mass | 515.603 g·mol−1 |
| |
| |
| (verify) | |
Nargenicin (CP-47,444, CS-682) is a 28 carbon macrolide with a fused tricyclic core that has in addition a unique ether bridge. The polyketide antibiotic was isolated from Nocardia argentinensis.[1] Nargenicin is effective towards gram-positive bacteria and been shown to have strong antibacterial activity against Staphylococcus aureus, including strains that are resistant to methicillin.[2] It has also been shown to induce cell differentiation and inhibit cell proliferation in a human myeloid leukemia cell line.[3]
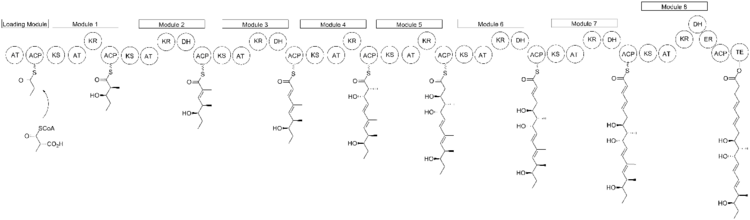
Biosynthesis
The biosynthesis of nargenicin is believed to be closely related to fatty acid biosynthesis to produce a polyketide chain. David E. Cane and colleagues have used feeding experiments to determine that nargenicin is derived from common precursors acetate and propionate.[4][5][6]
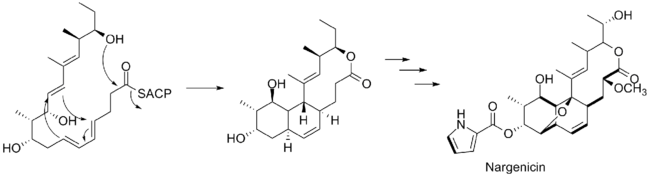
The polyketide chain produced then undergoes a ring closure to form the large lactone ring and a Diels–Alder reaction to form the fused cyclohexane/cyclohexene rings. The oxygen atoms attached to carbons in positions that do not correspond to polyketides—carbons 8 and 13 (the ether bridge), carbon 2 (the methoxy substituent), and carbon 18 (on the hydroxyethyl chain attached to the lactone ring) are derived from molecular oxygen.[7]
References
- ↑ "Structure of natural antibiotic CP-47,444". J. Am. Chem. Soc. 102 (12): 4203–4209. 1980. doi:10.1021/ja00532a036.
- ↑ "Production, isolation and biological activity of nargenicin from Nocardia sp. CS682". Archives of Pharmacal Research 31 (10): 1339–45. October 2008. doi:10.1007/s12272-001-2115-0. PMID 18958426.
- ↑ "Nargenicin enhances 1,25-dihydroxyvitamin D(3)- and all-trans retinoic acid-induced leukemia cell differentiation via PKCbetaI/MAPK pathways". Biochemical Pharmacology 77 (11): 1694–701. June 2009. doi:10.1016/j.bcp.2009.03.004. PMID 19428323.
- ↑ "Biosynthetic Origin of the Carbon Skeleton and Oxygen Atoms of Nargenicin A1". J. Am. Chem. Soc. 106 (3): 784–787. 1984. doi:10.1021/ja00315a052.
- ↑ "Macrolide Biosynthesis. 6 Mechanism of Polyketide Chain Elongation". Tetrahedron Lett. 32 (40): 5457–5460. 1991. doi:10.1016/0040-4039(91)80057-D.
- ↑ "Nargenicin Biosynthesis. Incorporation of Polyketide Chain Elongation Intermediates and Support for a Proposed Intramolecular Diels-Alder Cyclization". J. Am. Chem. Soc. 115 (2): 527–535. 1993. doi:10.1021/ja00055a024.
- ↑ "Nargenicin biosynthesis: late stage oxidations and absolute configuration". The Journal of Antibiotics 38 (3): 423–6. March 1985. doi:10.7164/antibiotics.38.423. PMID 4008333.
 |