Chemistry:Neoxaline
From HandWiki
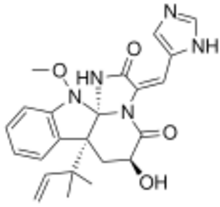
| |
| Names | |
|---|---|
| Other names
Nedoxaline
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| C23H25N5O4 | |
| Molar mass | 435.484 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Neoxaline is a bio-active Aspergillus japonicus isolate. It is an antimitotic agent and shows weak inhibitory activity of blood platelet aggregation. It weakly stimulates the central nervous system.[1][2] It has been synthesized through the "highly stereoselective introduction of a reverse prenyl group to create a quaternary carbon stereocenter using (−)-3a-hydroxyfuroindoline as a building block, construction of the indoline spiroaminal via cautious stepwise oxidations with cyclizations from the indoline, assembly of (Z)-dehydrohistidine, and photoisomerization of unnatural (Z)-neoxaline to the natural (E)-neoxaline."[3]
See also
References
- ↑ Neoxaline an antimiotic agent
- ↑ Hirano, A.; Iwai, Y.; Masuma, R.; Tei, K.; Omura, S. (August 1979). "Neoxaline, a new alkaloid produced by Aspergillus japonicus. Production, isolation and properties.". The Journal of Antibiotics 32 (8): 781-785. doi:10.7164/antibiotics.32.781. PMID 500498.
- ↑ Ideguchi, Tetsuya; Yamada, Takeshi; Shirahata, Tatsuya; Hirose, Tomoyasu; Sugawara, Akihiro; Kobayashi, Yoshinori; O̅mura, Satoshi; Sunazuka, Toshiaki (19 August 2013). "Asymmetric Total Synthesis of Neoxaline". Journal of the American Chemical Society 135 (34): 12568–12571. doi:10.1021/ja406657v. PMID 23957424.
 |
