Chemistry:Nierenstein reaction
| Nierenstein reaction | |
|---|---|
| Named after | Maximilian Nierenstein |
| Reaction type | Carbon-carbon bond forming reaction |
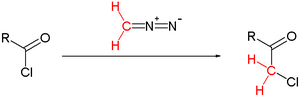
The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into a haloketone with diazomethane.[1][2] It is an insertion reaction in that the methylene group from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.
Reaction mechanism
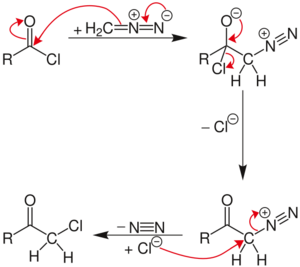
The reaction proceeds through a diazonium salt intermediate formed by nucleophilic acyl substitution of the chloride with diazomethyl anion. The chloride then displaces the diazo group in an SN2 reaction, with N2 as the leaving group.
If excess diazomethane is present during the reaction, it can act as a base, abstracting a hydrogen from the diazonium-salt intermediate. The result is a neutral diazoketone, which does not react with the chloride. Instead, the byproduct, diazonium-methyl from the other diazomethane molecule, can be attacked by the chloride to produce chloromethane. The unreactive diazoketone can be re-activated and reacted by treatment with hydrogen chloride to give the normal Nierenstein product.
File:Nierenstein reaction mechanism2.tif
In some cases, even limiting the amount of diazomethane gives a reaction process that stalls via the neutral diazoketone pathway, requiring the addition of HCl gas to rescue it.[3]
Scope
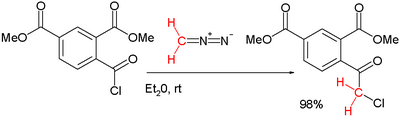
One original 1924 Nierenstein reaction:[4]
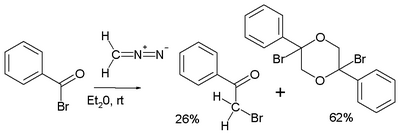
and a reaction starting from benzoyl bromide going haywire with formation of the dioxane dimer:[5]
See also
- Maximilian Nierenstein
- Curtius rearrangement
- Wolff rearrangement
- Arndt–Eistert reaction: where acid chlorides react with diazomethane to give chain extended carboxylic acids via a rearrangement
References
- ↑ Clibbens, D.; Nierenstein, M. (1915). "The action of diazomethane on some aromatic acyl chlorides". J. Chem. Soc. 107: 1491. doi:10.1039/CT9150701491. https://zenodo.org/record/1429737.
- ↑ Bachman, W. E.; Struve, W. S. (1942). "The Arndt-Eistert Reaction". Org. React. 1. (Review)
- ↑ McPhee, W. D; Klingsberg, E. Organic Syntheses, Coll. Vol. 3, p.119 (1955); Vol. 26, p.13 (1946). (Article)
- ↑ M. Nierenstein; D. G. Wang; J. C. Warr (1924). "The Action of Diazomethane on some Aromatic Acyl Chlorides II. Synthesis of Fisetol". J. Am. Chem. Soc. 46 (11): 2551–2555. doi:10.1021/ja01676a028.
- ↑ H. H. Lewis; M. Nierenstein; Enid M. Rich (1925). "The Action of Diazomethane on some Aromatic Acyl Chlorides III. The Mechanism of the Reaction". J. Am. Chem. Soc. 47 (6): 1728–1732. doi:10.1021/ja01683a036.
 |