Chemistry:Norcycloartenol
From HandWiki
| |
| Names | |
|---|---|
| Other names
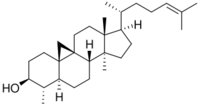
4α,14α-dimethyl-9β,19-cyclo-5α-cholest-24-en-3β-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C29H48O | |
| Molar mass | 412.702 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
29- or 31-Norcycloartenol[note 1], also called 4α,14α-dimethyl-9β,19-cyclo-5α-cholest-24-en-3β-ol, is a Metabolic intermediate of plant sterol biosynthesis.[1][2][3][4] In the pathway, it is transformed from demethylation of cycloartenol, then 9,19-cyclopropyl-ring opening reaction occurs to 29-Norlanosterol.[5]
Note
- ↑ zoology and botany are different in numbering of steroids side chain, 241 to 28, 242 to 29
References
- ↑ Zheng, S; Ma, Z; Ye, J; Wang, G; Wang, R; Zhou, H; Zeng, S; Jiang, H (1 January 2014). "Determination of three triterpene alcohols in rat plasma after oral administration of pollen of Brassica campestris based on the utilization of fetal bovine serum as surrogate matrix.". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 944: 11–7. doi:10.1016/j.jchromb.2013.10.044. PMID 24291607.
- ↑ Bellamine, A; Mangla, AT; Dennis, AL; Nes, WD; Waterman, MR (January 2001). "Structural requirements for substrate recognition of Mycobacterium tuberculosis 14 alpha-demethylase: implications for sterol biosynthesis.". Journal of Lipid Research 42 (1): 128–36. doi:10.1016/S0022-2275(20)32344-0. PMID 11160374.
- ↑ Bayer, M; Proksch, P; Felsner, I; Brenden, H; Kohne, Z; Walli, R; Duong, TN; Götz, C et al. (November 2011). "Photoprotection against UVAR: effective triterpenoids require a lipid raft stabilizing chemical structure.". Experimental Dermatology 20 (11): 955–8. doi:10.1111/j.1600-0625.2011.01350.x. PMID 21824200.
- ↑ Hartmann, MA; Perret, AM; Carde, JP; Cassagne, C; Moreau, P (8 August 2002). "Inhibition of the sterol pathway in leek seedlings impairs phosphatidylserine and glucosylceramide synthesis but triggers an accumulation of triacylglycerols.". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1583 (3): 285–96. doi:10.1016/s1388-1981(02)00249-4. PMID 12176396.
- ↑ "Rosa chinensis plant sterol biosynthesis II". https://pmn.plantcyc.org/RCHINENSIS/new-image?object=PWY-6663.
 |

