Chemistry:Obafluorin
From HandWiki
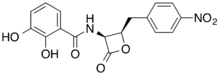
| |
| Names | |
|---|---|
| IUPAC name
2,3-dihydroxy-N-[(2R,3S)-2-[(4-nitrophenyl)methyl]-4-oxooxetan-3-yl]benzamide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H14N2O7 | |
| Molar mass | 358.306 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Obafluorin is a β-lactone antibiotic with the molecular formula C17H14N2O7.[1][2] Obafluorin is produced by the bacterium Pseudomonas fluorescens.[3] Obafluorin is a inhibitor of serine hydroxymethyltransferase.[4]
References
- ↑ Scott, Thomas A.; Batey, Sibyl F.; Wiencek, Patrick; Chandra, Govind; Alt, Silke; Franklyn, Christopher S.; Wilkinson, Barrie (17 July 2019). "Self-immunity guided identification of threonyl-tRNA synthetase as the molecular target of obafluorin, a β-lactone antibiotic". bioRxiv. doi:10.1101/704981.
- ↑ Tymiak, Adrienne A.; Culver, Catherine A.; Malley, Mary F.; Gougoutas, Jack Z. (December 1985). "Structure of obafluorin: an antibacterial β-lactone from Pseudomonas fluorescens". Journal of Organic Chemistry 50 (26): 5491–5495. doi:10.1021/jo00350a010.
- ↑ Wells, J. Scott; Trejo, William H.; Principe, Pacifico A.; Sykes, Richard B. (1984). "Obafluorin, a novel β-lactone produced by Pseudomonas fluorescens. Taxonomy, fermentation and biological properties.". Journal of Antibiotics 37 (7): 802–803. doi:10.7164/antibiotics.37.802. PMID 6432765.
- ↑ Prasad, Neha (18 April 2015). "Obafluorin: A Potential Inhibitor of Serine Hydroxymethyl Trasferase [sic"]. Undergraduate Research Symposium Posters. https://openscholarship.wustl.edu/undergrad_research/58/.
Further reading
- "Next antibiotic may come from dirt bacteria". Futurity. 2 August 2019.
- Scott, Thomas A.; Heine, Daniel; Qin, Zhiwei; Wilkinson, Barrie (26 June 2017). "An L-threonine transaldolase is required for L-threo-β-hydroxy-α-amino acid assembly during obafluorin biosynthesis" (in en). Nature Communications 8 (1): 15935. doi:10.1038/ncomms15935. ISSN 2041-1723. PMID 28649989.
- Herbert, Richard B.; Knaggs, Andrew R. (1992). "Biosynthesis of the antibiotic obafluorin from D-[U-13C]glucose and p-aminophenylalanine in Pseudomonas fluorescens" (in en). Journal of the Chemical Society, Perkin Transactions 1 (1): 103–107. doi:10.1039/P19920000103. ISSN 1364-5463.
 |

