Chemistry:Octasulfur monoxide
From HandWiki
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
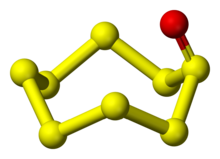
| S 8O | |
| Molar mass | 272.48 g·mol−1 |
| Appearance | Yellowish sharp crystal[1] |
| Density | 2.13 g·cm−3 |
| Solubility | 0.8 g[clarification needed](carbon disulfide)[1] |
| Structure[2] | |
| Orthorhombic | |
| Pca21 | |
a = 13.197, b = 7.973, c = 8.096
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octasulfur monoxide is an inorganic compound with a chemical formula S
8O, discovered in 1972. It is a type of sulfur oxide.[1]
A crystalline compound composed of cyclooctasulfur monoxide and antimony pentachloride in equimolar quantities can be made (S
8O · SbCl
5).[3]
References
- ↑ Jump up to: 1.0 1.1 1.2 Ralf Steudel, Michael Rebsch (April 1972). "Preparation of cyclo-Octasulfur Oxide, S8O" (in en). Angewandte Chemie International Edition in English 11 (4): 302–303. doi:10.1002/anie.197203021. ISSN 0570-0833.
- ↑ Steudel, Ralf; Luger, Peter; Bradaczek, Hans; Rebsch, Michael (May 1973). "Crystal and Molecular Structure of Cyclooctasulfur Oxide, S8O". Angewandte Chemie International Edition in English 12 (5): 423–424. doi:10.1002/anie.197304231.
- ↑ Steudel, Ralf; Sandow, Torsten; Steidel, Jürgen (1980). "Reversible isomerization of cyclo-octasulphur monoxide; preparation and X-ray crystal structure of S8O·SbCl5". J. Chem. Soc., Chem. Commun. (4): 180–181. doi:10.1039/C39800000180.
External links
 |

