Chemistry:Oltipraz
From HandWiki
Short description: Chemical compound
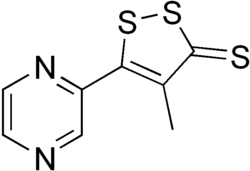 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C8H6N2S3 |
| Molar mass | 226.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Oltipraz is an organosulfur compound belonging to the dithiolethione class.[1][2] It acts as a schistosomicide and has been shown in rodent models to inhibit the formation of cancers in the bladder, blood, colon, kidney, liver, lung, pancreas, stomach, and trachea, skin, and mammary tissue.[3][4] Clinical trials of oltipraz have failed to demonstrate efficacy and have shown significant side effects, including neurotoxicity and gastrointestinal toxicity.[3] Oltipraz has also been shown to generate superoxide radicals, which can be toxic.[5]
References
- ↑ "Comparison of citrus coumarins on carcinogen-detoxifying enzymes in Nrf2 knockout mice". Toxicology Letters 185 (3): 180–186. March 2009. doi:10.1016/j.toxlet.2008.12.014. PMID 19150646.
- ↑ "Dithiolethiones: a privileged pharmacophore for anticancer therapy and chemoprevention". Future Medicinal Chemistry 10 (10): 1241–1260. May 2018. doi:10.4155/fmc-2017-0281. PMID 29749746.
- ↑ 3.0 3.1 "A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway". Molecular Cancer Therapeutics 3 (7): 885–893. July 2004. doi:10.1158/1535-7163.885.3.7. PMID 15252150.
- ↑ "Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis". Cancer Research 64 (18): 6424–6431. September 2004. doi:10.1158/0008-5472.CAN-04-1906. PMID 15374950.
- ↑ "Cancer chemopreventive oltipraz generates superoxide anion radical". Archives of Biochemistry and Biophysics 435 (1): 83–88. March 2005. doi:10.1016/j.abb.2004.11.028. PMID 15680910.
 |
