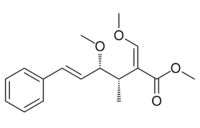
Chemistry:Oudemansin A
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (2E,3S,4R,5E)-4-methoxy-2-(methoxymethylidene)-3-methyl-6-phenylhex-5-enoate | |
| Other names
(−)-Oudemansin A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H22O4 | |
| Molar mass | 290.359 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oudemansin A is a natural product first isolated from the basidiomycete fungus Oudemansiella mucida. Its chemical structure was determined by X-ray crystallography in 1979 and absolute stereochemistry by total synthesis. Two closely related derivatives, oudemansin B and X have also been isolated from other basidiomycetes. They are all biologically active against many filamentous fungi and yeasts but with insufficient potency and stability to become useful commercial products. However, their discovery, together with the strobilurins led to agricultural fungicides including azoxystrobin with the same mechanism of action.[1]
Isolation and Characterization
Oudemansin A (initially known simply as oudemansin) with R1 = R2 = H was first described in 1979, after being isolated from mycelial fermentations of the basidiomycete fungus Oudemansiella mucida. Its structure, including the relative configuration of the methoxy and adjacent methyl groups, was established by both spectroscopic methods and single crystal X-ray analysis but its absolute stereochemistry was at that time undetermined.[2] Later it was found in cultures of the basidiomycete fungi Mycena polygramma and Xerula melanotricha. The latter fungus also produces oudemansin B, with R1 = MeO and R2 = Cl. Oudemansin X, with R1 = H and R2 = MeO was isolated from Oudemansiella radicata.[3]
Chemical synthesis
The oudemansins have been targets for total synthesis and in 1983, the synthesis of (-)-oudemansin A established that all three compounds have the (9S,10S)-configuration.[4] Routes to oudemansins B and X have also been reported.[1]
Mechanism of action as fungicides
The fungicidal effects were shown to stem from what was then a novel mode of action, QoI inhibition.[5] This was related to the β-methoxyacrylic acid sub-structure which this and related natural products, the strobilurins have in common. Intensive research by several agrochemical companies led to the development of useful agricultural fungicides based on the same mode of action, of which azoxystrobin is a typical example.[1]
References
- ↑ 1.0 1.1 1.2 Clough, J. M. (1993). "The strobilurins, oudemansins, and myxothiazols, fungicidal derivatives of β-methoxyacrylic acid". Natural Product Reports 10 (6): 565–574. doi:10.1039/NP9931000565. PMID 8121648.
- ↑ Anke, Timm; Hecht, Hans Jürgen; Chramm, Georgs; Steglich, Wolfgang (1979). "Antibiotics from basidiomycetes. IX. Oudemansin, an antifungal antibiotic from Oudemansiella mucida (Schrader ex Fr.) hoehnel (Agaricales)". The Journal of Antibiotics 32 (11): 1112–1117. doi:10.7164/antibiotics.32.1112. PMID 528381.
- ↑ Lorenzen, K.; Anke, T. (1998). "Basidiomycetes as a Source for New Bioactive Natural Products". Current Organic Chemistry (Bentham Science Publishers) 2 (4): 329–364. doi:10.2174/1385272802666220128213627. ISSN 1385-2728. https://books.google.com/books?id=aI_3BDcYKXgC&pg=PA329.
- ↑ Akita, Hiroyuki; Koshiji, Hiroko; Furuichi, Akiya; Horikoshi, Koki; Oishi, Takeshi (1983). "The absolute configuration of oudemansin total synthesis of (−)-oudemansin". Tetrahedron Letters 24 (19): 2009–2010. doi:10.1016/S0040-4039(00)81829-8.
- ↑ Becker, W.F.; von Jagow, G.; Anke, T.; Steglich, W. (1981). "Oudemansin, strobilurin A, strobilurin B and myxothiazol: New inhibitors of the bc 1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element". FEBS Letters 132 (2): 329–333. doi:10.1016/0014-5793(81)81190-8. PMID 6271595.
Further reading
- Anke, Timm; Steglich, Wolfgang (1999). "Strobilurins and Oudemansins". Drug Discovery from Nature. Springer. pp. 320–334. ISBN 3540648445.
- Morton, V.; Staub, T. (2008). "A Short History of Fungicides". Apsnet Feature Articles. doi:10.1094/APSnetFeature-2008-0308.
- Reddy, P.Parvatha (2013). "Chapter 12: Strobilurin fungicides". Recent advances in crop protection. Springer. pp. 185–200. doi:10.1007/978-81-322-0723-8. ISBN 978-81-322-0722-1. https://link.springer.com/chapter/10.1007/978-81-322-0723-8_12.
 |



