Chemistry:Ovothiol A
From HandWiki
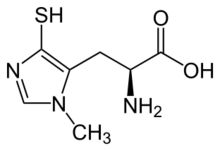
| |
| Names | |
|---|---|
| Systematic IUPAC name
(2S)-2-Amino-3-(1-methyl-4-sulfanyl-1H-imidazol-5-yl)propanoic acid | |
| Other names
1-N-Methyl-4-mercaptohistidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | C061475 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H11N3O2S | |
| Molar mass | 201.24 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ovothiol A (N1-methyl-4-mercaptohistidine) is a highly reducing antioxidant mercaptohistidine, which accumulates to very high levels in the eggs of certain marine invertebrates, including sea urchins, scallops and starfish,[1] where it acts to scavenge hydrogen peroxide released during fertilization.[2]
This thiol is also found in some human pathogens including trypanosomes and members of the genus Leishmania.[3]
It is synthesized by the addition and oxidation of cysteine to histidine by 5-histidylcysteine sulfoxide synthase, followed by methylation and further reduction.
References
- ↑ Turner, E.; Klevit, R.; Hager, L. J.; Shapiro, B. M. (1987). "Ovothiols, a family of redox-active mercaptohistidine compounds from marine invertebrate eggs". Biochemistry 26 (13): 4028–4036. doi:10.1021/bi00387a043. PMID 3651433.
- ↑ Turner, E.; Hager, L. J.; Shapiro, B. M. (1988). "Ovothiol replaces glutathione-peroxidase as a hydrogen-peroxide scavenger in sea-urchin eggs". Science 242 (4880): 939–941. doi:10.1126/science.3187533. PMID 3187533. Bibcode: 1988Sci...242..939T.
- ↑ Spies, H.S.; Steenkamp, D.J. (1994). "Thiols of intracellular pathogens. Identification of ovothiol A in Leishmania donovani and structural analysis of a novel thiol from Mycobacterium bovis". Eur. J. Biochem. 224 (1): 203–213. doi:10.1111/j.1432-1033.1994.tb20013.x. PMID 8076641.
 |

