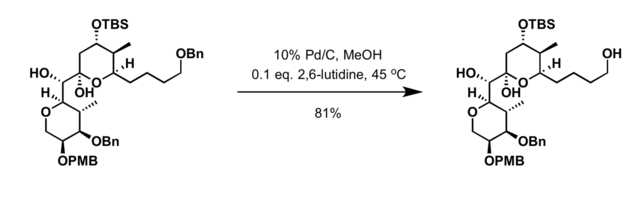Chemistry:Palladium on carbon

| |
| Names | |
|---|---|
| Other names
Palladium on carbon, Pd/C, Pd-C
| |
| Identifiers | |
| Properties | |
| Appearance | Black powder |
| Solubility | Aqua regia |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst.[1] The metal is supported on activated carbon to maximize its surface area and activity.
Uses
Hydrogenation
Palladium on carbon is used for catalytic hydrogenations in organic synthesis. Examples include reductive amination,[2] carbonyl reduction, nitro compound reduction,[3][4] the reduction of imines and Schiff bases[1] and debenzylation reactions.
Hydrogenolysis
Palladium on carbon is a common catalyst for hydrogenolysis. Such reactions are helpful in deprotection strategies. Particularly common substrates for hydrogenolysis are benzyl ethers:[5]
Other labile substituents are also susceptible to cleavage by this reagent. [6]
Coupling reactions
Palladium on carbon is also used for coupling reactions. Examples include the Suzuki reaction and Stille reaction.[7]
Preparation
A solution of palladium chloride and hydrochloric acid is combined with aqueous suspension of activated carbon. The palladium(II) is then reduced by the addition of formaldehyde.[8] Palladium loading is typically between 5% and 10%. Often the catalyst mixture is stored moist.
See also
- Palladium black
- Platinum on carbon
- Platinum dioxide
- Rhodium-platinum oxide
- Lindlar catalyst
- Raney nickel
- Urushibara nickel
References
- ↑ 1.0 1.1 Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 34–38. ISBN 9780471396987. https://books.google.com/books?id=RjZRAAAAMAAJ&q=0471396982.
- ↑ Romanelli, Michael G.; Becker, Ernest I. (1967). "Ethylp-dimethylaminophenylacetate". Organic Syntheses 47: 69. doi:10.15227/orgsyn.047.0069.
- ↑ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. p. 1816. ISBN 978-0-471-72091-1.
- ↑ Ram, Siya; Ehrenkaufer, Richard E. (1984). "A general procedure for mild and rapid reduction of aliphatic and aromatic nitro compounds using ammonium formate as a catalytic hydrogen transfer agent". Tetrahedron Letters 25 (32): 3415–3418. doi:10.1016/S0040-4039(01)91034-2.
- ↑ Smith, Amos B.; Zhu, Wenyu; Shirakami, Shohei; Sfouggatakis, Chris; Doughty, Victoria A.; Bennett, Clay S.; Sakamoto, Yasuharu (2003-03-01). "Total Synthesis of (+)-Spongistatin 1. An Effective Second-Generation Construction of an Advanced EF Wittig Salt, Fragment Union, and Final Elaboration". Organic Letters 5 (5): 761–764. doi:10.1021/ol034037a. ISSN 1523-7060. PMID 12605509.
- ↑ Musliner, Walter J.; Gates, Jr, John W. (1971). "Dehydroxylation of Phenols; Hydrogenolysis of Phenolic Ethers: Biphenyl". Organic Syntheses 51: 82. doi:10.15227/orgsyn.051.0082.
- ↑ Liebeskind, Lanny S.; Peña-Cabrera, Eduardo (2000). "Stille couplings catalyzed by palladium-on-carbon with CuI as a co-catalyst: synthesis of 2-(4'-Acetylhenyl)thiophene". Organic Syntheses 77: 138. doi:10.15227/orgsyn.077.0135.
- ↑ Mozingo, Ralph (1946). "Palladium catalysts". Organic Syntheses 26: 77–82. doi:10.15227/orgsyn.026.0077. PMID 20280763.
 |