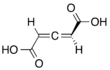
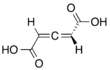
Chemistry:Penta-2,3-dienedioic acid
From HandWiki
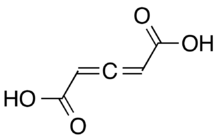
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Penta-2,3-dienedioic acid
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H4O4 | |||
| Molar mass | 128.083 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Penta-2,3-dienedioic acid (one of two chemicals called glutinic acid), is in allene-containing dicarboxylic acid. It was the first allene to be synthesized, in 1887, but the structure of it was thought to be a propyne core instead of an allene. The correct structural isomeric identity was not determined until 1954.[1]
Literature confusion
A diterpene, chemical name (4aR,5S,6R,8aR)-5-[(Z)-4-carboxy-3-methylbut-3-enyl]-5,6,8a-trimethyl-3,4,4a,6,7,8-hexahydronaphthalene-1-carboxylic acid (Template:Pubchem), is also called glutinic acid. Some database entries for "glutinic acid" incorrectly identify it as this diterpene rather than the allene meaning in the underlying publications.[2]
References
- ↑ Jones, E. R. H.; Mansfield, G. H.; Whiting, M. C. (1954-01-01). "Researches on acetylenic compounds. Part XLVII. The prototropic rearrangements of some acetylenic dicarboxylic acids" (in en). Journal of the Chemical Society (Resumed): 3208–3212. doi:10.1039/JR9540003208. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1954/jr/jr9540003208.
- ↑ See patents listed for Template:Pubchem
 |