Chemistry:Pentamethylcyclopentadienyl ruthenium dichloride dimer
| |
| Names | |
|---|---|
| IUPAC name
Di-μ-chlorido-bis[chlorido(pentamethyl-η5-cyclopentadienyl)ruthenium(III)]
| |
| Other names
Di-μ-chloro-bis[chloro(pentamethylcyclopentadienyl)ruthenium(III)]
Dichloro(pentamethylcyclopentadienyl)ruthenium(III) | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C20H30Cl4Ru2 | |
| Appearance | brown solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
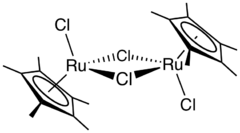
Pentamethylcyclopentadienyl ruthenium dichloride is an organoruthenium chemistry with the formula [(C5(CH3)5)RuCl2]2, commonly abbreviated [Cp*RuCl2]2. This brown paramagnetic solid is a reagent in organometallic chemistry. It is an unusual example of a compound that exists as isomers that differ in the intermetallic separation, a difference that is manifested in a number of physical properties.[1]
Preparation, structure, reactions
The compound has C2h symmetry, with each metal atom having pseudo-octahedral geometry. In the crystal structure, two isomers are observed in the unit cell, one with a 2.93 Å ruthenium–ruthenium bond and the other with a long internuclear distance of 3.75 Å. The former isomer is diamagnetic, and the latter is magnetic.[1][2]
It is prepared by the reaction of hydrated ruthenium trichloride with pentamethylcyclopentadiene.[3]
- 2 Cp*H + 2 RuCl3·3H2O → [Cp*RuCl2]2 + 2 HCl + 6 H2O
The reaction is accompanied by formation of decamethylruthenocene.
Pentamethylcyclopentadienyl ruthenium dichloride can reduced to the diamagnetic tetramer of Ru(II):
- 2 [Cp*RuCl2]2 + 2 Zn → [Cp*RuCl]4 + 2 ZnCl2
Methoxide also can be used to produce a related diruthenium(II) derivative, which is also diamagnetic:
- [Cp*RuCl2]2 + 3 NaOCH3 + HOCH3 → [Cp*RuOCH3]2] + 3 NaCl + CH2O + HCl
Treating the tetramer with 1,5-cyclooctadiene in etheral solvent gives the mononuclear complex chloro(1,5-cyclooctadiene)(pentamethylcyclopentadienyl)ruthenium(II).[4][5]
- 0.25 [Cp*RuCl]4 + 1,5-cyclooctadiene → Cp*RuCl(1,5-cyclooctadiene)
Compounds like Cp*RuCl(1,5-cyclooctadiene), the tetramer [Cp*RuCl]4, and related diamagnetic Cp*Ru(III) complexes have been investigated as hydrogenation catalysts.[6]
See also
- (Cymene)ruthenium dichloride dimer - [(cymene)RuCl2]2
- Pentamethylcyclopentadienyl iridium dichloride dimer - [Cp*IrCl2]2
References
- ↑ 1.0 1.1 McGrady, John E. (2000). "[(Cp*RuCl)2(μ-Cl)2]: bond-stretch or spin-state isomerism?". Angewandte Chemie International Edition 39 (17): 3077–3079. doi:10.1002/1521-3773(20000901)39:17<3077::AID-ANIE3077>3.0.CO;2-B. PMID 11028037.
- ↑ Kölle, Urich; Kossakowski, Janusz; Klaff, Norbert; Wesemann, Lars; Englert, Ulli; Herberich, Gerhard E. (1991). "Dichloro(pentamethylcyclopentadienyl)ruthenium—Novel Dichotomy in a Molecular Structure". Angew. Chem. Int. Ed. Engl. 30 (6): 690–691. doi:10.1002/anie.199106901.
- ↑ Kölle, Urich; Kossakowski, Janusz (1992). "Di-μ-Chloro-Bis[(η5 -Pentamethylcyclopentadienyl) Chlororuthenium(III)], [Cp* RuCl2 ]2 and Di-μ-methoxo-Bis(η5 -Pentamethylcyclopentadienyl)diruthenium(II), [Cp* RuOMe]2". Di-μ-Chloro-Bis[(η5-Pentamethylcyclopentadienyl) Chlororuthenium(III)], [Cp*RuCl2]2 and Di-μ-methoxo-Bis(η5-Pentamethylcyclopentadienyl)diruthenium(II), [Cp*RuOMe]2. Inorganic Syntheses. 29. pp. 225–228. doi:10.1002/9780470132609.ch52. ISBN 9780470132609.
- ↑ Koelle, Ulrigh; Kossakowski, Janusz (May 1989). "Pentamethylcyclopentadienylruthenium complexes". Journal of Organometallic Chemistry 362 (3): 383–398. doi:10.1016/0022-328x(89)87260-2. ISSN 0022-328X. https://doi.org/10.1016/0022-328X(89)87260-2.
- ↑ Fagan, Paul J.; Mahoney, Wayne S.; Calabrese, Joseph C.; Williams, Ian D. (June 1990). "Structure and chemistry of the complex tetrakis(.eta.5-pentamethylcyclopentadienyl)tetrakis(.mu.3-chloro)tetraruthenium(II): a useful precursor to (pentamethylcyclopentadienyl)ruthenium(0), -(II), and -(IV) complexes" (in en). Organometallics 9 (6): 1843–1852. doi:10.1021/om00156a025. ISSN 0276-7333. https://pubs.acs.org/doi/abs/10.1021/om00156a025.
- ↑ Fürstner, Alois (2019-01-09). "trans -Hydrogenation, gem -Hydrogenation, and trans -Hydrometalation of Alkynes: An Interim Report on an Unorthodox Reactivity Paradigm" (in en). Journal of the American Chemical Society 141 (1): 11–24. doi:10.1021/jacs.8b09782. ISSN 0002-7863. PMID 30422659. https://pubs.acs.org/doi/10.1021/jacs.8b09782.
 |
