Chemistry:Pentazine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentazine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| CHN5 | |
| Molar mass | 83.054 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
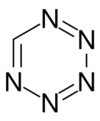
Pentazine is a hypothetical chemical compound that consists of a six-membered aromatic ring containing five nitrogen atoms with the molecular formula CHN5. The name pentazine is used in the nomenclature of derivatives of this compound.
Pentazine is predicted to be unstable and to decompose into hydrogen cyanide (HCN) and nitrogen (N2).[1] The activation energy required is predicted to be around 20 kJ/mol.[2]
See also
- 6-membered rings with one nitrogen atom: pyridine
- 6-membered rings with two nitrogen atoms: diazines
- 6-membered rings with three nitrogen atoms: triazines
- 6-membered rings with four nitrogen atoms: tetrazines
- 6-membered rings with six nitrogen atoms: hexazine
References
- ↑ Hurst, Derek T. (1996). "Other Tetrazines and Pentazines". Comprehensive Heterocyclic Chemistry II. pp. 957–965. doi:10.1016/B978-008096518-5.00138-6. ISBN 9780080965185.
- ↑ J. Fabian and E. Lewars (2004). "Azabenzenes (azines) — The nitrogen derivatives of benzene with one to six N atoms: Stability, homodesmotic stabilization energy, electron distribution, and magnetic ring current; a computational study". Canadian Journal of Chemistry 82 (1): 50–69. doi:10.1139/v03-178. Archived from the original on 2005-03-29. https://web.archive.org/web/20050329185413/http://pubs.nrc-cnrc.gc.ca/rp/rppdf/v03-178.pdf.
 |
