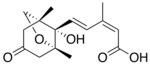
Chemistry:Phaseic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z,4E)-5-[(1R,5R,8S)-8-Hydroxy-1,5-dimethyl-3-oxo-6-oxabicyclo[3.2.1]octan-8-yl]-3-methylpenta-2,4-dienoic acid | |
| Other names
Phaseic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H20O5 | |
| Molar mass | 280.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phaseic acid is a terpenoid catabolite of abscisic acid. Like abscisic acid, it is a plant hormone associated with photosynthesis arrest[1] and abscission.
Function
Abscisic acid (ABA) is a multifunctional plant hormone, playing roles in germination, seasonal growth patterns, and stress response. ABA levels are believed to be regulated in part by control of ABA catabolism, specifically by oxidation to form phaseic acid.[2] Phaseic acid can therefore be thought of as a degradation product of ABA, although it may have other functions. The introduction of high phaseic acid concentrations have been found to impede stomatal closure and reduce photosynthesis in Arabidopsis[1] but this may be a result of product inhibition rather than recognition of phaseic acid by a receptor.
Phaseic acid inhibits glutamate receptors in mouse brain.[3]
Biosynthesis
Early precursors
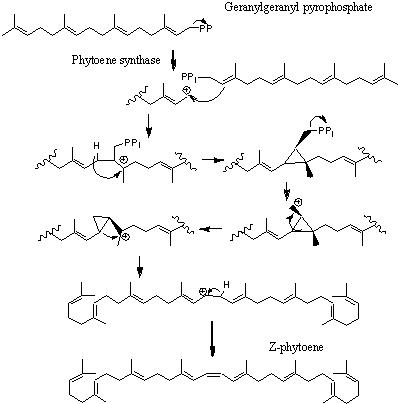
Phaseic acid is an isoprenoid, which means that it is derived from isoprene units. The activated terpene geranylgeranyl pyrophosphate is combined with itself to produce the common carotenoid precursor, lycopene.
Carotenoid precursors
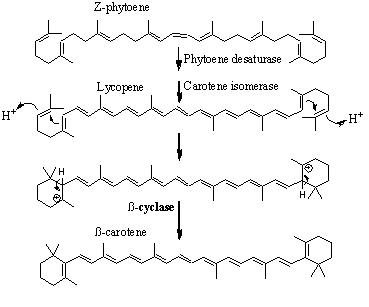
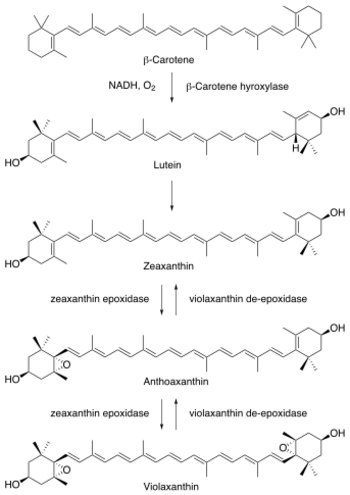
Phaseic acid is a product of abscisic acid, which is itself the product of the C40 carotenoid zeaxanthin via at least four enzymatic steps. Zeaxanthin is epoxidized to form violaxanthin or neoxanthin. The C15 end of the molecule is then cleaved by an epoxycarotenoid epoxygenase to form xanthoxin, an aldehyde.
Modification of xanthoxin
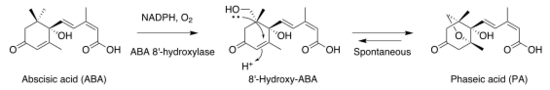
Xanthoxin is reduced at the epoxy group and then hydroxylated at the aldehyde group, producing abscisic acid.[4] The 8' hydroxylation of abscisate, abscisic acid's conjugate base, produces 8'-hydroxyabscisate. 8'-hydroxyabscisate cyclizes via nucleophilic attack of the existing ring by the 8' hydroxy group to interconvert with phaseate. The former process is known to be mediated by 8' abscisic acid hydroxylases, a family of NADPH-dependent enzymes. Saito et al. have demonstrated that, in the case of arabidopsis, these hydroxylases are independent of any regulatory mechanism downstream of translation itself.[5][6] The latter process is reported to occur without enzymatic intervention,[5][7] as it has been found to occur spontaneously in vitro.
- The biosynthesis of phaseic acid
References
- ↑ 1.0 1.1 Sharkey, T.D. "Effects of Phaseic Acid and Dihydrophaseic Acid on Stomata and the Photosynthetic Apparatus". Plant Physiol. 65(2): 291--297.
- ↑ Milborrow BV. (1969). "Identification of 'Metabolite C' from abscisic acid and a new structure for phaseic acid". Chem Commun 966-967.
- ↑ Hou, Sheng Tao; Jiang, Susan X.; Zaharia, L. Irina; Han, Xiumei; Benson, Chantel L.; Slinn, Jacqueline; Abrams, Suzanne R. (30 December 2016). "Phaseic Acid, an Endogenous and Reversible Inhibitor of Glutamate Receptors in Mouse Brain". Journal of Biological Chemistry 291 (53): 27007–27022. doi:10.1074/jbc.M116.756429. PMID 27864367.
- ↑ Cutler and Krochko (1999). Formation and breakdown of ABA. Trends in Plant Science, 4: 472-478
- ↑ 5.0 5.1 Saito S. (2004). "Arabidopsis CYP707As encode -abscisic acid 8'-hydroxylase, a key enzyme in the catabolism of abscisic acid". Plant Physiol. 134(4):1439-49.
- ↑ Kushiro T. (2004). "The arabidopsis cytochrome P450 CYP707A encodes ABA 8'-hydroxylases: key enzymes in ABA catabolism". EMBO J. 23(7):1647-56.
- ↑ Milborrow B.V. "The cyclization of 8'-hydroxy abscisic acid to phaseic acid in vivo". Phytochemistry 27:757-759.
 |