Chemistry:Phenylsilver
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H5Ag | |
| Molar mass | 184.974 g·mol−1 |
| Appearance | white solid[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
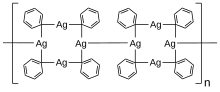
Phenylsilver is an organosilver compound with the chemical formula C6H5Ag. It is a white solid. The structure is a one-dimensional coordination polymer of silver(I) tetrads.[2]
Synthesis
It can be produced by treating silver nitrate with trialkylphenyltin (or trialkylphenyllead at −10 °C). In the presence of excess silver nitrate, the reaction produces the bright yellow complex C6H5Ag·2AgNO3.[1] Phenylsilver can also be produced by treating silver nitrate with diphenylzinc in a 1:2 molar ratio and 0 °C. If the molar ratio is 1:1, it will produce orange C6H5Ag·2AgNO3.[3] Earlier syntheses using phenyl magnesium bromide will produce phenylsilver that contains silver or magnesium salt impurities, which destabilize it.[3][4]
When heating phenylsilver in a speed of 5 °C/min, it decomposes at 67 °C. If the heating speed is 10 °C/min, it will explode at 47 °C.[1]
- 2 C6H5Ag → C6H5-C6H5 + 2 Ag[3]
References
- ↑ 1.0 1.1 1.2 Beverwijk, C. D. M.; van der Kerk, G. J. M. (1972). "The synthesis and properties of phenylsilver". Journal of Organometallic Chemistry 43 (1): C11–C12. doi:10.1016/S0022-328X(00)81760-X.
- ↑ Lenz, Tabea; Hebenbrock, Marian (2024). "Phenylsilver – an unexpected one-dimensional coordination polymer of silver(i) tetrads". Dalton Transactions 53: 423–427. doi:10.1039/D3DT03007E.
- ↑ 3.0 3.1 3.2 J. Boersma; F.J.A. Des Tombe; F. Weijers; G.J.M. Van Der Kerk (January 1977). "A new, easy synthesis of phenylsilver" (in en). Journal of Organometallic Chemistry 124 (2): 229–233. doi:10.1016/S0022-328X(00)90970-7. https://linkinghub.elsevier.com/retrieve/pii/S0022328X00909707. Retrieved 2020-12-09.
- ↑ Reich, Rene. New organometallic compounds: Copper phenyl and silver phenyl. Compt. rend., 1923. 177: 322-324. CAN18: 2618.
Further reading
- Nicole J. Rijs, Richard A. J. O’Hair (2010-05-24). "Unimolecular Reactions of Organocuprates and Organoargentates" (in en). Organometallics 29 (10): 2282–2291. doi:10.1021/om1000875. ISSN 0276-7333. https://pubs.acs.org/doi/abs/10.1021/om1000875. Retrieved 2020-12-09.
- John P. Fackler (2011-12-15). "Silver: Organometallic Chemistry". in Robert A. Scott (in en). Encyclopedia of Inorganic and Bioinorganic Chemistry. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/9781119951438.eibc0206. ISBN 9781119951438. http://doi.wiley.com/10.1002/9781119951438.eibc0206. Retrieved 2020-12-09.
External links
 |

