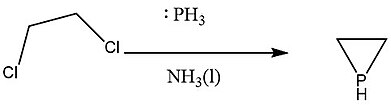Chemistry:Phosphiranes
Phosphiranes are organic compounds with the phosphirane functional group – a three-membered ring with two atoms of carbon and one atom of phosphorus that has lots of ring angle strain. Phosphiranes are usually synthesized by double substitution reactions or pericyclic pathways. Phosphiranes can also be oxidized into phosphirane oxides, undergo SN2 substitution reactions, or decompose into different units.
Structure
Phosphirane functional group is a very strained structure - the C-P-C bond angle in phosphirane ring structure is 49°,[1] even lower than the C-N-C angle in aziridine and the C-C-C angle in cyclopropane (60°). This high angle strain causes a higher inversion barrier as well as the increased s-character of the lone pair on the phosphorus atom, making the phosphorus atom a very weak nucleophile. As a result, there is no known phosphiranium salt with the phosphirane structure protonated on the phosphorus atom.
Synthesis
Double bimolecular nucleophilic substitution of vicinal dichlorides
The first synthesis of phosphirane was done by reacting sodium phosphinide with 1,2-dichloroethane in liquid ammonia.[2] In this case, the phosphinide anion, acting as the strong nucleophile, substitutes one of the chlorides first and then substitutes the other one intramolecularly, both of which are through SN2 mechanism.
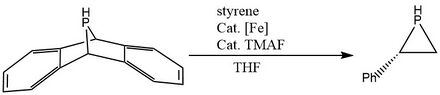
Phosphinidene transfer to electron deficient olefins
The very recent synthesis[3] reported in 2019 proceeds through the transfer of a phosphinidene species from dibenzo-7-(R)-7-phospha-norbornadiene (RPA) onto styrene under the catalysis of tetramethylammonium fluoride and [Fe(η5-C5H5)(CO)2(THF)][BF4], forming an enantiomerically pure phosphirane.
In 2022, reported by Tiansi Xin, the same reaction was achieved[4] using a different catalyst: [CpFe(CO)2]2.
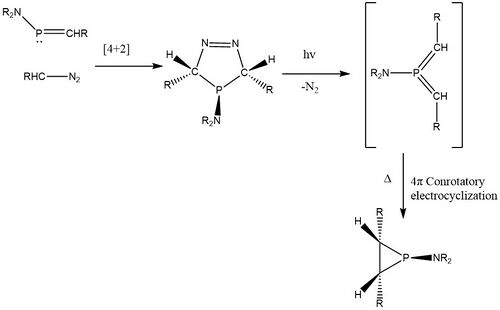
Combining phosphaalkenes and diazomethane species through cycloaddition
Besides synthesizing phosphiranes from P and C2 units, phosphiranes can also be produced from PC and C units, such as the reaction between of bis(trimethylsilyl)-aminotrimethylsilylmethylenephosphine and trimethylsilyldiazomethane.[5] Upon photon irritation, the dinitrogen group of the cycloaddition product leaves, generating a phosphorus (V) intermediate that undergoes electrocyclization to give the phosphorane functional group.
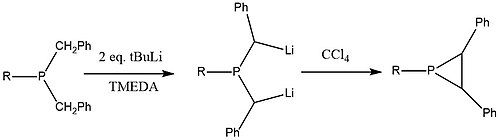
Cyclization of tertiary phosphines and phosphine oxides derivatives
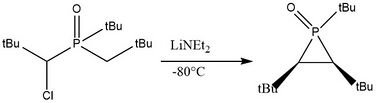
Tertiary dibenzylphosphine is treated with 2 equivalents of tert-butyllithium with tetramethylethylenediamine to generate the dilithium intermediate which cyclizes to phosphirane upon addition of carbon tetrachloride,[6]
Similarly, phosphirane oxides can be generated from the corresponding bisylide derivatives[7]using lithium diethylamide:
Note that the t-butyl or other bulky functional groups are needed to ensure the stability of the product.
Polycyclic phosphirane synthesis
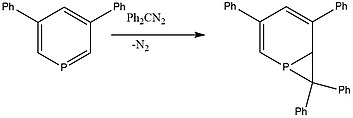
Phosphorine can react with diphenyldiazomethane to generate polycyclic phosphirane species.[8]
The diazomethane species must be highly conjugated to ensure the formation of the three membered ring.
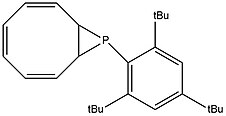
By reacting dilithium cyclooctatetraenide with 2,4,6-tri-t-butylphenylphosphonous dichloride, the following air and room temperature stable bicyclic phosphirane can be produced with the help of steric protection from the bulky ligand:[9]
Reactivity
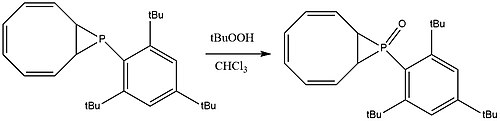
The phosphirane species on the 8-membered ring compound mentioned in the previous paragraph can be oxidized into the corresponding phosphirane oxide by t-butyl hydroperoxide.[9] Note that the product, which is stable under room temperature and air, still contains a sterically strained 3-membered ring due to steric protection from the nearby bulky ligands.
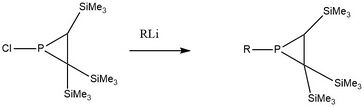
Since the lone pair of phosphorus atom in the phosphirane has an increased amount of s-character, phosphirane's nucleophilicity is low but its electrophilicity is high. Therefore, one can substitute the chloride in the following sterically hindered phosphirane chloride using different corresponding alkyllithium reagents.[10]
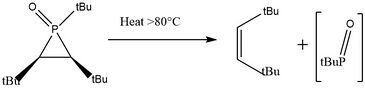
Upon heating, the t-butyl group protected phospirane oxide species decomposes into (Z)-2,2,5,5-tetramethyl-3-hexene and a very reactive intermediate t-butyl phosphinidene oxide which can be further trapped using water, methanol, or o-quinones.[11]
References
- ↑ Mathey, Francois (1990-09-01). "Chemistry of 3-membered carbon-phosphorus heterocycles" (in en). Chemical Reviews 90 (6): 997–1025. doi:10.1021/cr00104a004. ISSN 0009-2665. https://pubs.acs.org/doi/abs/10.1021/cr00104a004.
- ↑ Wagner, Ross I.; Freeman, LeVern D.; Goldwhite, H.; Rowsell, D. G. (1967-03-01). "Phosphiran" (in en). Journal of the American Chemical Society 89 (5): 1102–1104. doi:10.1021/ja00981a013. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00981a013.
- ↑ Geeson, Michael B.; Transue, Wesley J.; Cummins, Christopher C. (2019-08-28). "Organoiron- and Fluoride-Catalyzed Phosphinidene Transfer to Styrenic Olefins in a Stereoselective Synthesis of Unprotected Phosphiranes" (in en). Journal of the American Chemical Society 141 (34): 13336–13340. doi:10.1021/jacs.9b07069. ISSN 0002-7863. PMID 31408599.
- ↑ Xin, Tiansi; Geeson, Michael B.; Zhu, Hui; Qu, Zheng-Wang; Grimme, Stefan; Cummins, Christopher C. (2022-11-09). "Synthesis of phosphiranes via organoiron-catalyzed phosphinidene transfer to electron-deficient olefins" (in en). Chemical Science 13 (43): 12696–12702. doi:10.1039/D2SC05011K. ISSN 2041-6539. PMID 36519032.
- ↑ Niecke, Edgar; Leuer, Martina; Wildbredt, Dirk-A.; Schoeller, Wolfgang W. (1983). "A stereoselective route from a dihydro-1,2,4λ 3 -diazaphosphole to a λ 3 -phosphirane via a bismethylenephosphorane intermediate" (in en). J. Chem. Soc., Chem. Commun. (20): 1171–1172. doi:10.1039/C39830001171. ISSN 0022-4936. http://xlink.rsc.org/?DOI=C39830001171.
- ↑ Kolodyazhnaya, O. O.; Kolodyazhnyi, O. I. (2015-02-01). "Synthesis of phosphiranes" (in en). Russian Journal of General Chemistry 85 (2): 436–440. doi:10.1134/S1070363215020140. ISSN 1608-3350. https://doi.org/10.1134/S1070363215020140.
- ↑ Makar, A. B.; McMartin, K. E.; Palese, M.; Tephly, T. R. (1975-06-01). "Formate assay in body fluids: application in methanol poisoning". Biochemical Medicine 13 (2): 117–126. doi:10.1016/0006-2944(75)90147-7. ISSN 0006-2944. PMID 1. https://pubmed.ncbi.nlm.nih.gov/1.
- ↑ Märkl, Gottfried; Beckh, Hans J.; Mayer, Klaus K.; Ziegler, Manfred L.; Zahn, Thomas (1987-03-01). "Reaction of Phosphinines with Diazoalkanes: Diphosphachiropteradienes by Intramolecular, Ionic 5s + 5s [6 + 4 Cycloaddition"]. Angewandte Chemie International Edition in English 26 (3): 236–238. doi:10.1002/anie.198702361. ISSN 0570-0833. http://dx.doi.org/10.1002/anie.198702361.
- ↑ Jump up to: 9.0 9.1 Quin, Louis D; Yao, En-Yun; Szewczyk, Jerzy (1987-01-01). "Special Properties Imparted to the 9-Phosphabicyclo[6.1.0nonatriene system by a P-(2,4,6-tri-t-butylphenyl) substituent; 17O NMR spectrum of a bicyclic phosphirane oxide"]. Tetrahedron Letters 28 (10): 1077–1080. doi:10.1016/s0040-4039(00)95915-x. ISSN 0040-4039. http://dx.doi.org/10.1016/s0040-4039(00)95915-x.
- ↑ Niecke, Edgar; Leuer, Martina; Nieger, Martin (1989-03-01). "Synthese und Reaktivität eines 1‐Chlor‐λ3‐phosphirans". Chemische Berichte 122 (3): 453–461. doi:10.1002/cber.19891220310. ISSN 0009-2940. http://dx.doi.org/10.1002/cber.19891220310.
- ↑ Quast, Helmut; Heuschmann, Manfred (1982-03-01). "tert-Butylphosphinidenoxid und (Z)-2,2,5,5-Tetramethyl-3-hexen durch stereospezifische thermische [2 + 1-Cycloeliminierung aus r-1,t-2,t-3-Tri-tert-butylphosphiran-1-oxid"]. Chemische Berichte 115 (3): 901–909. doi:10.1002/cber.19821150307. ISSN 0009-2940. http://dx.doi.org/10.1002/cber.19821150307.
 |