Chemistry:Polythionates
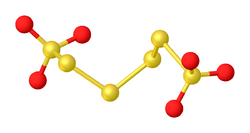
Polythionates are oxyanions with the formula −
O
3S–S
n–SO−
3 (n ≥ 0). They occur naturally and are the products of redox reactions of thiosulfate. Polythionates are readily isolable, unlike the parent polythionic acids.[2]
Preparation
Many members of the polythionates have been characterized: dithionate, trithionate, tetrathionate, pentathionate, etc.
These salts are often generated by oxidation of thiosulfate. For example, tetrathionate is obtained by oxidation of thiosulfate ion with iodine (reaction is used in iodometry):
- S
2O2−
3 + I
2 → S
4O2−
6 + 2 I−
More specialized routes involve reactions of sulfur chlorides with bisulfite salts:
- SCl
2 + 2 HSO−
3 → [O
3SSSO
3]2− + 2 HCl - S
2Cl
2 + 2 HSO−
3 → [O
3SS
2SO
3]2− + 2 HCl - SCl
2 + 2 HS
2O−
3 → [O
3SS
3SO
3]2− + 2 HCl
Potassium pentathionate ion has been obtained from SCl
2, sodium thiosulfate, and potassium acetate. Initially prismatic crystals of potassium tetrathionate appear, then lamellar crystals of potassium pentathionate, from which the influence of tartaric acid makes an aqueous solution of pentathionic acid.[3]
Potassium hexathionate K
2S
6O
6 has been synthesized by combining KNO
2 and K
2S
2O
3 in concentrated HCl at low temperatures.[4]
References
- ↑ Marøy, Kjartan (1973). "Refinement of the Crystal Structure of trans-Dichlorobis(ethylenediamine)cobalt(III) Hexathionate Monohydrate". Acta Chemica Scandinavica 27: 1705–1716. doi:10.3891/acta.chem.scand.27-1705.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ P. W. Schenk (1963). "Sulfur, Selenium, Tellurium". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 401.
- ↑ P. W. Schenk (1963). "Sulfur, Selenium, Tellurium". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 403.
 |
