Chemistry:Polyvinylidene chloride
| |
| Names | |
|---|---|
| IUPAC name
Poly(1,1-dichloroethene)[1]
| |
| Other names
Poly(vinylidene dichloride)
Polydene | |
| Identifiers | |
| Abbreviations | PVDC |
| ChemSpider |
|
| Properties | |
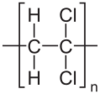
| (C2H2Cl2)n | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polyvinylidene chloride, or polyvinylidene dichloride (PVDC), is a homopolymer of vinylidene chloride.
History
Ralph Wiley accidentally discovered polyvinylidene chloride polymer in 1933.[2] At the time he was a college student who worked part-time at Dow Chemical lab as a dishwasher.[3] While cleaning laboratory glassware, he came across a vial he could not scrub clean. Dow researchers made this material into a greasy, dark green film,[4] first called "Eonite" and then "Saran".[3][5]
Ralph Wiley went on to become one of Dow Chemical's research scientists and invent and develop many plastics, chemicals and production machines.
The military sprayed Saran on fighter planes to guard against salty sea spray, and carmakers used it for upholstery. Dow later devised a formulation of polyvinylidene chloride free of unpleasant odour and green colour.
The most well known use of polyvinylidene chloride came in 1953, when Saran Wrap, a plastic food wrap, was introduced. In 2004, however, the formula was changed to low-density polyethylene due to environmental concerns about its chlorine content and other disadvantages.
Properties
It is a remarkable barrier against water, oxygen, and aromas. It has a superior chemical resistance to alkalis and acids, is insoluble in oil and organic solvents, has a very low moisture regain and is impervious to mold, bacteria, and insects. It is soluble in polar solvents.
Above 125 °C, it decomposes to produce HCl.[6]
Disadvantages
While extremely useful as a food packaging agent, the major disadvantage of polyvinylidene chloride is that it will undergo thermally induced dehydrochlorination at temperatures very near to processing temperatures. This degradation easily propagates, leaving polyene sequences long enough to absorb visible light and change the color of the material from colorless to an undesirable transparent brown (unacceptable for one of polyvinylidene chloride's chief applications: food packaging). Therefore, there is a significant amount of product loss in the manufacturing process, which increases production and consumer costs.
Fiber types
Saran fiber is produced in monofilament, multifilament-twist, and staple fiber forms. It is also available in thermochromic (color changing) and luminescent (glow in the dark) fibers.
Uses
Packaging
Polyvinylidene chloride is applied as a water-based coating to films made of other plastics, such as biaxially-oriented polypropylene (BOPP) and polyethylene terephthalate (PET). This coating increases the barrier properties of the film, reducing the permeability of the film to oxygen and flavours and thus extending the shelf life of the food inside the package. It can also impart a high-gloss finish, which may be aesthetically pleasing and also provides a high degree of scuff resistance if applied over print.
Other
In household settings, PVDC is used in cleaning cloths, filters, screens, tape, shower curtains, and garden furniture. Industrially, it is used in screens, artificial turf, waste-water treatment materials, and underground materials. PVDC is also used in doll hair, stuffed animals, fabrics, fishnet, pyrotechnics, and shoe insoles. It was used in special photographic film.
Trademarks (producers)
- Saran TC and Saran LS (Asahi-Kasei)
- (formerly) Saran Wrap and Saranex (Dow Chemical)
- Ixan and Diofan (SolVin)
- SK Saran
See also
References
- ↑ "Vinylidene Chloride". https://pubchem.ncbi.nlm.nih.gov/compound/6366.
- ↑ John W. Klooster (30 July 2009). Icons of Invention: The Makers of the Modern World from Gutenberg to Gates. ABC-CLIO. pp. 466–. ISBN 978-0-313-34743-6. https://books.google.com/books?id=WKuG-VIwID8C&pg=PA466. Retrieved 12 July 2012.
- ↑ 3.0 3.1 David John Cole; Eve Browning; Fred E. H. Schroeder (30 April 2003). Encyclopedia of Modern Everyday Inventions. Greenwood Publishing Group. p. 129. ISBN 978-0-313-31345-5. https://books.google.com/books?id=rVQfBSlAZvAC&pg=PA129. Retrieved 12 July 2012.
- ↑ Andrew F. Smith (1 May 2007). The Oxford Companion to American Food and Drink. Oxford University Press. p. 492. ISBN 978-0-19-530796-2. https://books.google.com/books?id=AoWlCmNDA3QC&pg=PT492. Retrieved 12 July 2012.
- ↑ Mary Bellis. "Saran Wrap ®". inventors.about.com. http://inventors.about.com/library/inventors/blsaranwrap.htm.
- ↑ Otto G. Piringer; A. L. Baner (17 September 2008). Plastic Packaging: Interactions with Food and Pharmaceuticals. John Wiley & Sons. pp. 41–. ISBN 978-3-527-62143-9. https://books.google.com/books?id=wxvTeGVK5o8C&pg=PA41. Retrieved 12 July 2012.
- B.A. Howell, J. Polym. Sci., Polym. Chem. (ed) 1987, 25, 1681–1695.
- B.A. Howell, B.S. Warner, C.V. Rajaram, S.I. Ahemed and Z. Ahmed, Polym. Adv. Technol., 1994, 5, 485.
- B.A. Howell and S. M. Jane, “Impact of Moisture on the Thermal Stability of Vinylidene Chloride Copolymers”, Proceedings, 34th Annual Meeting of the North American Thermal Analysis Society, 2006.
- R.A. Wessling, D.S. Gibbs P.T. Delassus, B.E. Obi, B.A. Howell, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley and Sons, New York, 4th Edition, 1997, Vol 24, pp. 883–923.
External links
 |

