Chemistry:Potassium azodicarboxylate
From HandWiki
| |
| Names | |
|---|---|
| Other names
Potassium azocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2K2N2O4 | |
| Molar mass | 194.229 g·mol−1 |
| Appearance | Yellow crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
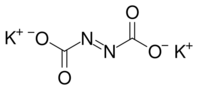
Potassium azodicarboxylate is a chemical compound with the formula C2K2N2O4. This chemical is used as a precursor to diimide. It can be synthesized by the reaction of potassium hydroxide with azodicarbonamide and it reacts with carboxylic acids to form diimide.[1]
References
- ↑ Pasto, Daniel J. (2001). "Potassium Azodicarboxylate" (in en). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289x.rp195. ISBN 9780470842898.
 |
