Chemistry:Potassium tetracarbonyliron hydride
From HandWiki
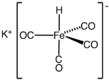
| |
| Names | |
|---|---|
| Other names
potassium tetracarbonylhydroferrate(1-)
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4HFeKO4 | |
| Molar mass | 207.991 g·mol−1 |
| Appearance | yellow solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Potassium tetracarbonyliron hydride is the inorganic salt with the formula K[HFe(CO)4]. A pale yellow solid, it is the potassium salt of [HFe(CO)4]−, which is the conjugate base of iron tetracarbonyl dihydride:[1][2]
- H2Fe(CO)4 + KOH → K[HFe(CO)4] + H2O
Potassium tetracarbonyliron hydride is prepared by treating iron pentacarbonyl with potassium hydroxide:[3]
- Fe(CO)5 + 2 KOH → K[HFe(CO)4] + KHCO3
Potassium tetracarbonyliron hydride is an intermediate in the synthesis of trisubstituted phosphine complexes:[4]
- K[HFe(CO)4] + 3 PBu3 + EtOH → Fe(CO)2(PBu3)3 + EtOK + 2 CO + H2
References
- ↑ Brunet, Jean-Jacques (1998). "Coordination Chemistry of Mononuclear Iron Carbonyl Complexes". Coordination Chemistry Reviews 178–180: 331–351. doi:10.1016/S0010-8545(98)00075-7.
- ↑ Brunet, J.J. (1990). "Tetracarbonylhydridoferrates, MHFe(CO)4: Versatile tools in Organic Synthesis and Catalysis". Chem. Rev. 90 (6): 1041–1059. doi:10.1021/cr00104a006.
- ↑ Brunet, J.-J.; Kindela, F. B.; Neibecker, D. (1992). "Trans-Tricarbonylbis(Phosphine)Iron(0) Complexes: One-Pot Syntheses from Pentacarbonyliron". trans-Tricarbonylbis(Phosphine)Iron(0) Complexes: One-Pot Syntheses from Pentacarbonyliron. Inorganic Syntheses. 29. pp. 151–156. doi:10.1002/9780470132609.ch36. ISBN 9780470132609.
- ↑ Brunet, J.-J.; Kindela, F. B.; Neibecker (1996). "One-Pot Synthesis of Dicarbonyltris(Phosphane)Iron(0) Complexes from Pentacarbonyliron". Inorganic Syntheses. Inorganic Syntheses. 31. pp. 202–210. doi:10.1002/9780470132623.ch30. ISBN 9780470132623.
 |

