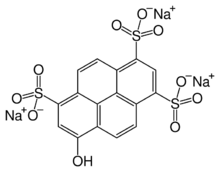
Chemistry:Pyranine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Trisodium 8-hydroxypyrene-1,3,6-trisulfonate
| |
| Other names
8-Hydroxypyrene-1,3,6-trisulfonic acid; Solvent Green 7; HPTS; Sulfonated hydroxy pyrene trisodium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H7Na3O10S3 | |
| Molar mass | 524.37 g·mol−1 |
| Appearance | Yellow-green crystalline powder |
| Soluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyranine is a hydrophilic, pH-sensitive fluorescent dye from the group of chemicals known as arylsulfonates.[2][3] Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator.[4][5] One example would be the measurement of intracellular pH.[6] Pyranine is also found in yellow highlighters, giving them their characteristic fluorescence and bright yellow-green colour. It is also found in some types of soap.[7]
Synthesis
It is synthesized from pyrenetetrasulfonic acid and a solution of sodium hydroxide in water under reflux.[8] The trisodium salt crystallizes as yellow needles when adding an aqueous solution of sodium chloride.
See also
References
- ↑ "C&L Inventory". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/32949.
- ↑ "chem industry entry". http://www.chemindustry.com/chemicals/1196265.html.
- ↑ "Comparative Toxicogenomics Database entry". http://ctdbase.org/detail.go?type=chem&acc=C005047.
- ↑ "chemical land 21 entry". http://chemicalland21.com/lifescience/foco/PYRANINE.htm.
- ↑ "Sci-Toys entry". http://sci-toys.com/scichem/jqp000/4136521.html.
- ↑ "Loading pyranine via purinergic receptors Bing by Siang Gan". http://ajpcell.physiology.org/cgi/content/abstract/275/4/C1158.
- ↑ "D&C Green No. 8 (C.I. 59040)". https://www.whatsinproducts.com/chemicals/view/1/115. Retrieved 2023-05-04.
- ↑ Tietze, Ernst; Bayer, Otto (1939). "Die Sulfosäuren des Pyrens und ihre Abkömmlinge". Justus Liebig's Annalen der Chemie 540 (1): 189–210. doi:10.1002/jlac.19395400113. ISSN 0075-4617.
External links
 |

