Chemistry:Pyrrolostatin
From HandWiki
| |
| Names | |
|---|---|
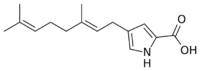
| IUPAC name
4-[(2E)-3,7-Dimethylocta-2,6-dienyl]-1H-pyrrole-2-carboxylic acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H21NO2 | |
| Molar mass | 247.338 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pyrrolostatin is a lipid peroxidation inhibitor with the molecular formula C15H21NO2 which has been isolated from the bacterium Streptomyces chrestomyceticus.[1][2]
References
- ↑ 1.0 1.1 "Pyrrolostatin" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Pyrrolostatin#section=3D-Conformer.
- ↑ Kato, Shinichiro; Shindo, Kazutoshi; Kawai, Hiroyuki; Odagawa, Atsuo; Matsuoka, Michiko; Mochizuki, Junichiro (1993). "Pyrrolostatin, a novel lipid peroxidation inhibitor from Streptomyces chrestomyceticus. Taxonomy, fermentation, isolation, structure elucidation and biological properties.". The Journal of Antibiotics 46 (6): 892–899. doi:10.7164/antibiotics.46.892. PMID 8344870.
Further reading
- Schmidt, Jens; Adrian, Juliane; Stark, Christian B. W. (22 July 2015). "Short and highly efficient synthesis of lipid peroxidation inhibitor pyrrolostatin and some analogues thereof" (in en). Organic & Biomolecular Chemistry 13 (30): 8173–8176. doi:10.1039/C5OB01066G. ISSN 1477-0539. PMID 26154919.
- Dunford, Damian G.; Knight, David W. (22 June 2016). "A brief total synthesis of Pyrrolostatin" (in en). Tetrahedron Letters 57 (25): 2746–2748. doi:10.1016/j.tetlet.2016.05.010. ISSN 0040-4039.
 |

