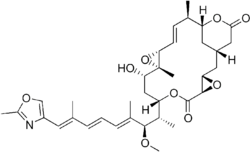
Chemistry:Rhizoxin
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C35H47NO9 | |
| Molar mass | 625.749 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhizoxin is an antimitotic agent with anti-tumor activity.[1][2] It is isolated from a pathogenic plant fungus (Rhizopus microsporus) which causes rice seedling blight.
Biosynthesis
Rhizoxin is biosynthesised by Paraburkholderia rhizoxinica, a bacterial endosymbiont of the fungus Rhizopus microsporus.[3] It is one of a large group of rhizoxin-like compounds produced by the bacteria.[4] The bacterial endosymbiont can be grown independently in culture. This may allow easy harvesting of rhizoxin and the related compounds avoiding total chemical synthesis, although total chemical synthesis is possible.[5]
Cytotoxic function
Rhizoxin binds beta tubulin in eukaryotic cells disrupting microtubule formation. This, in turn, prevents formation of the mitotic spindle inhibiting cell division. Additionally rhizoxin can depolymerise assembled microtubules.[6] The function of rhizoxin is similar to Vinca alkaloids.
Rhizoxin has undergone clinical trials as an anti-cancer drug[7] although it did not reach later stages of clinical trials due to low activity in vivo. Related compounds to rhizoxin may have improved biological activity.[4]
Structure
Rhizoxin is a 16-membered lactone ring connected to an oxazole ring by a long unsaturated chain.[8]
References
- ↑ "Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines". Cancer Research 46 (1): 381–385. January 1986. PMID 3753552.
- ↑ "In vitro evaluation of antimicrotubule agents in human small-cell lung cancer cell lines". Anticancer Research 19 (5B): 3985–8. 1999. PMID 10628341.
- ↑ "Pathogenic fungus harbours endosymbiotic bacteria for toxin production". Nature 437 (7060): 884–8. October 2005. doi:10.1038/nature03997. PMID 16208371. Bibcode: 2005Natur.437..884P.
- ↑ 4.0 4.1 "Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus". Journal of the American Chemical Society 128 (35): 11529–36. September 2006. doi:10.1021/ja062953o. PMID 16939276.
- ↑ Mitchell, I.S. (2005). "A total synthesis of the antitumour macrolide rhizoxin D". Org. Biomol. Chem. 3 (24): 4412–31. doi:10.1039/b507570j. PMID 16327903.
- ↑ "Studies on macrocyclic lactone antibiotics. XI. Anti-mitotic and anti-tubulin activity of new antitumor antibiotics, rhizoxin and its homologues". The Journal of Antibiotics 40 (1): 66–72. January 1987. doi:10.7164/antibiotics.40.66. PMID 3606749.Erratum in J. Antibiot. (Tokyo)., 40 (4), following 565. (1987)
- ↑ "Multicentre phase II pharmacological evaluation of rhizoxin. Eortc early clinical studies (ECSG)/pharmacology and molecular mechanisms (PAMM) groups". British Journal of Cancer 74 (12): 1944–8. December 1996. doi:10.1038/bjc.1996.657. PMID 8980394.
- ↑ "Studies on macrocyclic lactone antibiotics. VII. Structure of a phytotoxin "rhizoxin" produced by Rhizopus chinensis". The Journal of Antibiotics 37 (4): 354–62. April 1984. doi:10.7164/antibiotics.37.354. PMID 6547134.
 |

