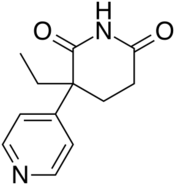
Chemistry:Rogletimide
 | |
| Clinical data | |
|---|---|
| Other names | Roglethimide; Pyridoglutethimide |
| Routes of administration | By mouth |
| Drug class | Aromatase inhibitor |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H14N2O2 |
| Molar mass | 218.256 g·mol−1 |
| 3D model (JSmol) | |
| |
Rogletimide, also known as pyridoglutethimide, is a medication which was never marketed.[1] It is related in chemical structure to the sedative/hypnotic drug glutethimide, but instead has pharmacological activity as a selective aromatase inhibitor similar to the related drug aminoglutethimide and has no significant sedative-hypnotic effect.[2] This makes it potentially useful in the treatment of breast cancer, and with fewer side effects than aminoglutethimide, but its lower potency caused it to be unsuccessful in clinical trials.[1][3]
Synthesis

Base catalyzed alkylation of ethyl 4-pyridylacetate [54401-85-3] (1) with iodoethane gives ethyl 2-(4-pyridyl)butyrate [76766-56-8] (2). Base catalyzed conjugate addition of the carbanion to acrylamide (3) gives (4). The last step is an intramolecular cyclization to rogletimide (5).
References
- ↑ 1.0 1.1 Frederick A. Luzzio (17 May 2019). Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry. Elsevier Science. pp. 361–. ISBN 978-0-12-815676-6. https://books.google.com/books?id=awKZDwAAQBAJ&pg=PA361.
- ↑ "Aromatase inhibitors--mechanisms for non-steroidal inhibitors". Breast Cancer Research and Treatment 30 (1): 43–55. 1994. doi:10.1007/bf00682740. PMID 7949204.
- ↑ "The influence of aminoglutethimide and its analogue rogletimide on peripheral aromatisation in breast cancer". British Journal of Cancer 66 (4): 692–7. October 1992. doi:10.1038/bjc.1992.339. PMID 1419608.
- ↑ Boss, Aileen M.; W. Clissold, Derek; Mann, John; Markson, Andrew J.; Thickitt, Christopher P. (1989). "A concise synthesis of racemic pyridoglutethimide and its resolution using chiral stationary phase HPLC". Tetrahedron. 45 (18): 6011–6016. doi:10.1016/S0040-4020(01)89128-6.
- ↑ Derek W. Clissold, John Mann, Christopher P. Thickitt, US5112976 (1992 to National Research Development Corporation).
 |
