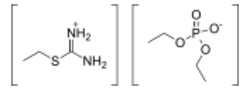
Chemistry:S-Ethylisothiouronium diethylphosphate
 | |
| Clinical data | |
|---|---|
| Trade names | Difetur |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C7H19N2O4PS |
| Molar mass | 258.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
S-Ethylisothiouronium diethylphosphate (brand name Difetur) is an S-alkylisothiouronium derivative used as an antihypotensive drug. The S-alkylisothiouronium compounds are used in processes of treating acute hypotension, which may result, for example, from shock or hemorrhage, and in processes for treating hyperoxic conditions, for example, oxygen poisoning.[1]
Indications
It is used for increasing arterial blood pressure in cases of acute arterial hypotension due to surgical interference, trauma, poisoning, shock condition, hemorrhages; in conjunction with epidural anesthesia; in overdose of ganglion blockers, alpha-adrenergic blockers, neuroleptics, anesthetics; and in other conditions when adrenomimetics are contra-indicated or ineffective. Difetur was also found to possess oxygen protective activity and, thus, can be used as a medicament for protecting against oxygen poisoning conditions caused by hyperoxia.[1]
S-Ethylisothiouronium diethylphosphate is also used for the treatment of headaches, in particular, migraines, as well as for the prevention or treatment of nausea and vomiting, effective in preventing or alleviating emesis associated with migraines or other medical conditions such as chemotherapy or radiotherapy, as well as other symptoms of migraines including phonophobia and photophobia.[2]
Mechanism of action
S-Ethylisothiouronium diethylphosphate is a specific inhibitor of inducible NO synthase on hepatic NO production level.[3][4][5] S-Ethylisothiouronium diethylphosphate affects systemic hemodynamic indices in the following way: the peripheral vascular resistance increases, the stroke volume and central blood volume increase, and the work of the left ventricle improves. Other effects are: analgesic, uterotonic,[6] decongestive[7] and anti-inflammatory.[8]
In difetur’s antihypotensive effect unlike sympathomimetics there is no stimulation of the sympathetic system, thus does not cause tachycardia, nor alter the acid-base balance and electrolyte and does not cause hypotension secondary. These drugs have also sedative action, reduce body temperature and oxygen consumption.[citation needed]
Forms
Injectable solution 10%-1 ml in ampules N 10.[citation needed]
References
- ↑ Jump up to: 1.0 1.1 "Pharmaceutical Compositions Comprising S-Alkylisothiouronium Derivatives" WO patent application 9813036, published 2 April 1998
- ↑ Barkan R, Mirimsky A, "Pharmaceutical compositions for headache, migraine, nausea and emesis", US patent application 7148208, published 8 January 2004
- ↑ "Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas". The Journal of Biological Chemistry 269 (43): 26669–76. October 1994. PMID 7523409. http://www.jbc.org/content/269/43/26669.full.pdf.
- ↑ "[The estimation of the nitric oxide role in the aggravation of combined radiation-thermal injuries]" (in ru). Radiatsionnaia Biologiia, Radioecologiia 45 (3): 316–9. 2005. PMID 16080623.
- ↑ "Nitric Oxide Level Increasing and Hyperfibrinogenemia in the Pathogenesis of Combined Radiation-Thermal Injuries". Cytokines & Inflammation (1). 2006. http://www.cytokines.ru/english/2006/1/Art11.php.
- ↑ Barkan R, Ghicavii V, "S-Alkylisothiouronium Derivatives for Treating Abnormal Uterine Bleeding Disorders", US patent application 2010305210, published 2 December 2010, assigned to Barkan Pharma SRL
- ↑ Sandu, Andrei-Victor. EUROINVENT 2009. Exhibition Catalogue. ISBN 978-973-702-641-5. http://eudirect.ro/euroinvent/cat/e2009.pdf.
- ↑ "S-Alkylisothiouronium Derivatives for the Treatment of Inflammatory Diseases" WO patent application 2007108004, published 27 September 2007, assigned to Barkan Pharma SRL
 |

