Chemistry:Sakurai reaction
| Sakurai reaction | |
|---|---|
| Named after | Hideki Sakurai Akira Hosomi |
| Reaction type | Addition reaction |
| Identifiers | |
| Organic Chemistry Portal | hosomi-sakurai-reaction |
| RSC ontology ID | RXNO:0000443 |
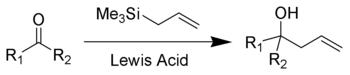
The Sakurai reaction (also known as the Hosomi–Sakurai reaction) is the chemical reaction of carbon electrophiles (such as a ketone shown here) with allyltrimethylsilane catalyzed by strong Lewis acids.[1][2][3]
Lewis acid activation is essential for complete reaction. Strong Lewis acids such as titanium tetrachloride, boron trifluoride, tin tetrachloride, and AlCl(Et)2 are all effective in promoting the Hosomi reaction. The reaction is a type of electrophilic allyl shift with formation of an intermediate beta-silyl carbocation. Driving force is the stabilization of said carbocation by the beta-silicon effect.
The Hosomi-Sakurai reaction can be performed on a number of functional groups. An electrophilic carbon, activated by a Lewis acid, is required. Below is a list of different functional groups that can be used in the Hosomi–Sakurai reaction. The reaction achieves results similar to the addition of an allyl Grignard reagent to the carbonyl.
Mechanism
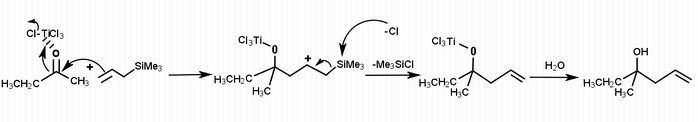
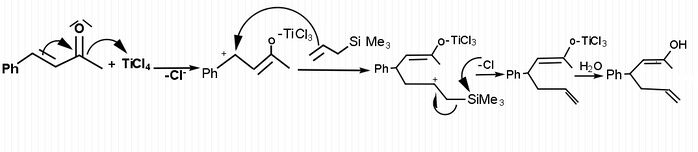
[4] The Hosomi-Sakurai reactions are allylation reactions which involve use of allyl silanes as allylmetal reagents. This section demonstrates examples of allylation of diverse ketones. In figure 1, allylation of a carbonyl ketone (compound containing a ketone group and two different functional groups) has been shown. In the given reaction, the electrophilic compound (carbon with a ketone group) is treated with titanium tetrachloride, a strong Lewis acid and allyltrimethylsilane. According to the general principle, the Lewis acid first activates the electrophilic carbon in presence of allyltrimethylsilane which then undergoes nucleophilic attack from electrons on the allylic silane.[5] The silicon plays the key role in stabilizing the carbocation of carbon at the β-position. Hosomi-Sakurai reaction is also applicable for other functional groups such as enones, where conjugate addition is usually seen. In figure 2, the Hosomi- Sakurai reaction has been shown using a cinnamoyl ketone. This reaction follows the same mechanism as the previous reaction shown here.
Beta-silicon effect stabilization
As displayed in the mechanism, the Hosomi–Sakurai reaction goes through a secondary carbocation intermediate. Secondary carbocations are inherently unstable, however the β-silicon effect from the silicon atom stabilizes the carbocation. Silicon is able to donate into an empty p-orbital, and the silicon orbital is shared between the two carbons. This stabilizes the positive charge over 3 orbitals. Another term for the β-silicon effect is silicon-hyperconjugation. This interaction is essential for the reaction to go to completion.
Literature of historic interest
- Sakurai, Hideki; Hosomi, Akira; Kumada, Makoto (1969). "Addition of trichloromethyl radicals to alkenylsilanes". The Journal of Organic Chemistry 36 (4): 1764–1768. doi:10.1021/jo01258a052.
- Hosomi, Akíra; Sakurai, Hideki (1976). "Syntheses of γ,δ-unsaturated alcohols from allylsilanes and carbonyl compounds in the presence of titanium tetrachloride". Tetrahedron Letters 17 (16): 1295–1298. doi:10.1016/S0040-4039(00)78044-0. ISSN 0040-4039.
- (Hosomi, Akira; Endo, Masahiko; Sakurai, Hideki (1976-09-05). "Allylsilanes as synthetic intermediates. ii. syntheses of homoallyl ethers from allylsilanes and acetals promoted by titanium tetrachloride". Chemistry Letters 5 (9): 941–942. doi:10.1246/cl.1976.941. ISSN 0366-7022.
- Hosomi, Akira; Sakurai, Hideki (1977-03-01). "Chemistry of organosilicon compounds. 99. Conjugate addition of allylsilanes to .alpha.,.beta.-enones. A New method of stereoselective introduction of the angular allyl group in fused cyclic .alpha.,.beta.-enones". Journal of the American Chemical Society 99 (5): 1673–1675. doi:10.1021/ja00447a080. ISSN 0002-7863.
References
- ↑ Hosomi, Akira (1988-05-01). "Characteristics in the reactions of allylsilanes and their applications to versatile synthetic equivalents". Accounts of Chemical Research 21 (5): 200–206. doi:10.1021/ar00149a004. ISSN 0001-4842.
- ↑ Fleming, Ian; Dunoguès, Jacques; Smithers, Roger (2004), "The Electrophilic Substitution of Allylsilanes and Vinylsilanes" (in en), Organic Reactions (American Chemical Society): pp. 57–575, doi:10.1002/0471264180.or037.02, ISBN 9780471264187
- ↑ Fleming, Ian (1991-01-01). "Allylsilanes, Allylstannanes and Related Systems". in Trost, Barry M.; Fleming, Ian. 2.2 – Allylsilanes, Allylstannanes and Related Systems. Pergamon. pp. 563–593. doi:10.1016/b978-0-08-052349-1.00041-x. ISBN 9780080523491. http://www.sciencedirect.com/science/article/pii/B978008052349100041X.
- ↑ "Hosomi-Sakurai Reaction". https://www.organic-chemistry.org/namedreactions/hosomi-sakurai-reaction.shtm.
- ↑ Yamasaki, Shingo; Fujii, Kunihiko; Wada, Reiko; Kanai, Motomu; Shibasaki, Masakatsu (2002-06-01). "A General Catalytic Allylation Using Allyltrimethoxysilane". Journal of the American Chemical Society 124 (23): 6536–6537. doi:10.1021/ja0262582. ISSN 0002-7863. PMID 12047165.
External links
- Hosomi-Sakurai reaction @ www.organic-chemistry.org Link
- Akira Hosomi HP
 |