Chemistry:Grignard reagent
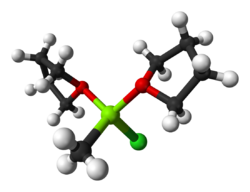
Grignard reagents or Grignard compounds are chemical compounds with the general formula RMgX(S)
n, where X is a halide, R is an organic group (normally an alkyl or aryl), S is an ether, and n is usually 2. Usually, the ether groups are omitted from the formula. Thus, two typical examples are methylmagnesium chloride ClMgCH
3 and phenylmagnesium bromide C
6H
5MgBr. They are a subclass of the organomagnesium compounds.
Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. The carbon-magnesium bond in Grignard reagent is a polar covalent bond. The carbon atom has negative excess charge and acts as a nucleophile.
Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complexes with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands.
The discovery of the Grignard reaction in 1900 was recognized with the Nobel Prize awarded to Victor Grignard in 1912.
Synthesis
From Mg metal
Traditionally Grignard reagents are prepared by treating an organic halide (normally organobromine) with magnesium metal. Ethers are required to stabilize the organomagnesium compound. Water and air, which rapidly destroy the reagent by protonolysis or oxidation, are excluded.[1] Although the reagents still need to be dry, ultrasound can allow Grignard reagents to form in wet solvents by activating the magnesium such that it consumes the water.[2]
As is common for reactions involving solids and solution, the formation of Grignard reagents is often subject to an induction period. During this stage, the passivating oxide on the magnesium is removed. After this induction period, the reactions can be highly exothermic. This exothermicity must be considered when a reaction is scaled-up from laboratory to production plant.[3] Most organohalides will work, but carbon-fluorine bonds are generally unreactive, except with specially activated magnesium (through Rieke metals).
Magnesium
Typically the reaction to form Grignard reagents involves the use of magnesium ribbon. All magnesium is coated with a passivating layer of magnesium oxide, which inhibits reactions with the organic halide. Many methods have been developed to weaken this passivating layer, thereby exposing highly reactive magnesium to the organic halide. Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, and sonication.[4] Iodine, methyl iodide, and 1,2-dibromoethane are common activating agents. The use of 1,2-dibromoethane is advantageous as its action can be monitored by the observation of bubbles of ethylene. Furthermore, the side-products are innocuous:
- Mg + BrC
2H
4Br → C
2H
4 + MgBr
2
The amount of Mg consumed by these activating agents is usually insignificant. When treated with small amounts of mercuric chloride, magnesium pieces become coated with an amalgam, enhancing its reactivity.
Addition of preformed Grignard reagent is often used as the initiator and oxidiser
Specially activated magnesium, such as Rieke magnesium, circumvents this problem.[5] The oxide layer can also be broken up using ultrasound, using a stirring rod to scratch the oxidized layer off,[6] or by adding a few drops of iodine or 1,2-Diiodoethane. Another option is to use sublimed magnesium or magnesium anthracene.[7]
"Rieke magnesium" is prepared by a reduction of an anhydrous magnesium chloride with a potassium:
- MgCl
2 + 2 K → Mg + 2 KCl
Mechanism
In terms of mechanism, the reaction proceeds through single electron transfer:[8][9][10]
Mg transfer reaction (halogen–Mg exchange)
An alternative preparation of Grignard reagents involves transfer of Mg from a preformed Grignard reagent to an organic halide. Other organomagnesium reagents are used as well.[11] This method offers the advantage that the Mg transfer tolerates many functional groups. An illustrative reaction involves isopropylmagnesium chloride and aryl bromide or iodides:[12]
- i-PrMgCl + ArBr → i-PrCl + ArMgBr
From alkylzinc compounds (reductive transmetalation)
A further method to synthesize Grignard reagents involves reaction of Mg with an organozinc compound. This method has been used to make adamantane-based Grignard reagents, which are, due to C-C coupling side reactions, difficult to make by the conventional method from the alkyl halide and Mg. The reductive transmetalation achieves:[13]
- AdZnBr + Mg → AdMgBr + Zn
Organomagnesium fluorides
Grignard reagents with chloride, bromide, and iodide are routine reagents. The corresponding fluorides RMgF were not synthesized until 1970.[14] In 1920 Swarts reported the reduction of amyl fluoride to the corresponding hydrocarbon with activated magnesium,[15] while no intermediates were separated. Alkylmagnesium fluoride was first prepared by Ashly and co-workers in 1970, using metal magnesium and catalytic iodine in refluxing tetrahydrofuran or 1,2-dimethoxyethane from the corresponding alkyl fluoride.[16][17]
Testing Grignard reagents
Because Grignard reagents are so sensitive to moisture and oxygen, many methods have been developed to test the quality of a batch. Typical tests involve titrations with weighable, anhydrous protic reagents, e.g. menthol in the presence of a color-indicator. The interaction of the Grignard reagent with phenanthroline or 2,2'-biquinoline causes a color change.[18]
Reactions of Grignard reagents
| Grignard reagent reactions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | Victor Grignard | ||||||||||
| Reaction type | Coupling reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
As nucleophiles
Grignard reagents react with a variety of carbonyl derivatives.[19]
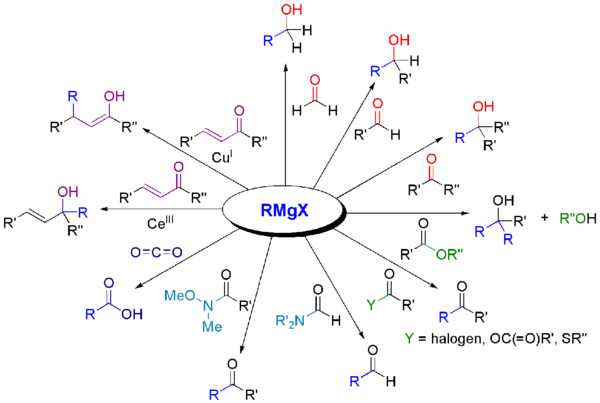
Such reactions usually involve an aqueous acidic workup, though this step is rarely shown in reaction schemes.
The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e. the Grignard reaction:[20]

3C(=O)CH(OCH
3)
2 with H
2C=CHMgBr
Note that the acetal functional group (a protected carbonyl) does not react.
Grignard reagents also react with many "carbonyl-like" electrophiles:
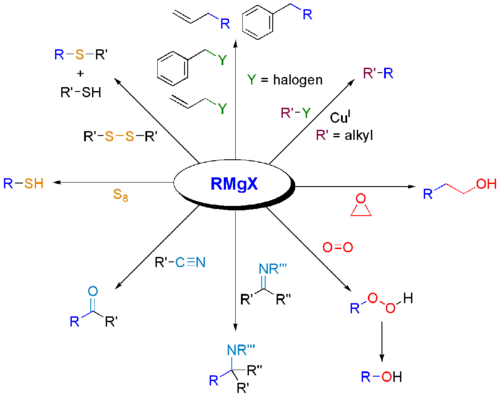
Compounds with labile protons are unsuitable electrophiles, because Grignard reagents are strong bases, and protonative quenching occurs much faster than addition.
Grignard reagents are nucleophiles in nucleophilic aliphatic substitutions for instance with alkyl halides in a key step in industrial Naproxen production:
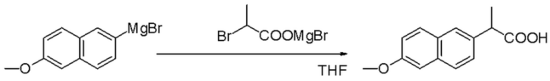
In the Bruylants reaction, a nitrile can be replaced by the Grignard nucleophile, rather than the Grignard attacking the nitrile to form an imino structure.[21]
Reactions as a base
Grignard reagents are basic and react with alcohols, phenols, etc. to give alkoxides (ROMgBr).[22] 1,3-Diketones and related substrates are also acidic enough that the Grignard reagent RMgX functions merely as a base, liberating the alkane RH to give a magnesium enolate.
Alkylation of metals and metalloids
Like organolithium compounds, Grignard reagents usefully form carbon–heteroatom bonds with many metal-based electrophiles. For example, they undergo transmetallation with cadmium chloride (CdCl2) to give dialkylcadmium:[23]
- 2 RMgX + CdCl
2 → R
2Cd + 2 Mg(X)Cl
Schlenk equilibrium
Most Grignard reactions are conducted in ethereal solvents, especially diethyl ether and THF. Grignard reagents react with 1,4-dioxane to give the diorganomagnesium compounds and insoluble coordination polymer MgX
2(dioxane)
2 and (R = organic group, X = halide):
- 2 RMgX + dioxane ⇌ R
2Mg + MgX
2(dioxane)
2
This reaction exploits the Schlenk equilibrium, driving it toward the right.
Precursors to magnesiates
Grignard reagents react with organolithium compounds to give ate complexes (Bu = butyl):[24]
- BuMgBr + 3 BuLi → LiMgBu
3 + BuBr
Coupling with organic halides
Grignard reagents do not typically react with organic halides, in contrast with their high reactivity with other main group halides. In the presence of metal catalysts, however, Grignard reagents participate in C-C coupling reactions. For example, nonylmagnesium bromide reacts with methyl p-chlorobenzoate to give p-nonylbenzoic acid, in the presence of Tris(acetylacetonato)iron(III) (Fe(acac)3), after workup with NaOH to hydrolyze the ester, shown as follows. Without the Fe(acac)3, the Grignard reagent would attack the ester group over the aryl halide.[25]

For the coupling of aryl halides with aryl Grignard reagents, nickel chloride in tetrahydrofuran (THF) is also a good catalyst. Additionally, an effective catalyst for the couplings of alkyl halides is the Gilman catalyst lithium tetrachlorocuprate (Li
2CuCl
4), prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl
2) in THF. The Kumada-Corriu coupling gives access to (substituted) styrenes.
Oxidation
Treatment of a Grignard reagent with oxygen gives the magnesium organoperoxide.[citation needed] Hydrolysis of this material yields hydroperoxides or alcohol. These reactions involve radical intermediates.
The simple oxidation of Grignard reagents to give alcohols is of little practical importance as yields are generally poor. In contrast, two-step sequence via a borane (vide supra) that is subsequently oxidized to the alcohol with hydrogen peroxide is of synthetic utility.
The synthetic utility of Grignard oxidations can be increased by a reaction of Grignard reagents with oxygen in presence of an alkene to an ethylene extended alcohol.[26] This modification requires aryl or vinyl Grignards. Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential. The only drawback is the requirement of at least two equivalents of Grignard although this can partly be circumvented by the use of a dual Grignard system with a cheap reducing Grignard such as n-butylmagnesium bromide.
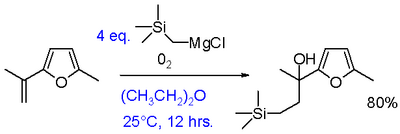
Elimination
In the Boord olefin synthesis, the addition of magnesium to certain β-haloethers results in an elimination reaction to the alkene. This reaction can limit the utility of Grignard reactions.
Allyl Grignard reagents
Allyl Grignard reagents exhibit high reactivity and special selectivity compared to alkyl ones.[27][28]
Industrial use
An example of the Grignard reaction is a key step in the (non-stereoselective) industrial production of Tamoxifen[29] (currently used for the treatment of estrogen receptor positive breast cancer in women):[30]
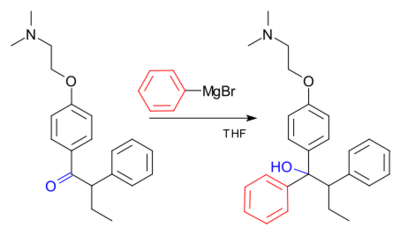
See also
Gallery
-
Magnesium turnings are placed in a flask.
-
Tetrahydrofuran and a small piece of iodine are added.
-
A solution of alkyl bromide is added while heating.
-
After completion of the addition, the mixture is heated for a while.
-
Formation of the Grignard reagent is complete. A small amount of magnesium still remains in the flask.
-
The Grignard reagent thus prepared is cooled to 0°C before the addition of the carbonyl compound. The solution becomes cloudy as the Grignard reagent precipitates out.
-
A solution of carbonyl compound is added to the Grignard reagent.
-
The solution is warmed to room temperature. At this point the reaction is complete.
References
- ↑ Goebel, M. T.; Marvel, C. S. (1933). "The Oxidation of Grignard Reagents". Journal of the American Chemical Society 55 (4): 1693–1696. doi:10.1021/ja01331a065. Bibcode: 1933JAChS..55.1693G.
- ↑ Smith, David H. (1999). "Grignard Reactions in "Wet" Ether". Journal of Chemical Education 76 (10): 1427. doi:10.1021/ed076p1427. Bibcode: 1999JChEd..76.1427S.
- ↑ Philip E. Rakita (1996). "5. Safe Handling Practices of Industrial Scale Grignard Ragents". Handbook of Grignard reagents. CRC Press. pp. 79–88. ISBN 0-8247-9545-8. https://books.google.com/books?id=82CaxfY-uNkC&pg=PA80.
- ↑ Smith, David H. (1999). "Grignard Reactions in "Wet" Ether". Journal of Chemical Education 76 (10): 1427. doi:10.1021/ed076p1427. Bibcode: 1999JChEd..76.1427S.
- ↑ Rieke, R. D. (1989). "Preparation of Organometallic Compounds from Highly Reactive Metal Powders". Science 246 (4935): 1260–1264. doi:10.1126/science.246.4935.1260. PMID 17832221. Bibcode: 1989Sci...246.1260R.
- ↑ Clayden, Jonathan; Greeves, Nick (2005). Organic chemistry. Oxford: Oxford Univ. Press. pp. 212. ISBN 978-0-19-850346-0. https://archive.org/details/organicchemistry00clay_0/page/212.
- ↑ Wakefield, Basil J. (1995). Organomagnesium Methods in Organic Chemistry. Academic Press. pp. 21–25. ISBN 0080538177.
- ↑ Garst, J. F.; Ungvary, F. "Mechanism of Grignard reagent formation". In Grignard Reagents; Richey, R. S., Ed.; John Wiley & Sons: New York, 2000; pp 185–275. ISBN 0-471-99908-3.
- ↑ Advanced Organic chemistry Part B: Reactions and Synthesis F.A. Carey, R.J. Sundberg 2nd Ed. 1983. Page 435
- ↑ Garst, J.F.; Soriaga, M.P. "Grignard reagent Formation", Coord. Chem. Rev. 2004, 248, 623 - 652. doi:10.1016/j.ccr.2004.02.018.
- ↑ Arredondo, Juan D.; Li, Hongmei; Balsells, Jaume (2012). "Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions". Organic Syntheses 89: 460. doi:10.15227/orgsyn.089.0460.
- ↑ Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. (2003). "Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange". Angewandte Chemie International Edition 42 (36): 4302–4320. doi:10.1002/anie.200300579. PMID 14502700.
- ↑ Armstrong, D.; Taullaj, F.; Singh, K.; Mirabi, B.; Lough, A. J.; Fekl, U. (2017). "Adamantyl Metal Complexes: New Routes to Adamantyl Anions and New Transmetallations". Dalton Transactions 46 (19): 6212–6217. doi:10.1039/C7DT00428A. PMID 28443859.
- ↑ Jagirdar, Balaji R.; Murphy, Eamonn F.; Roesky, Herbert W. (1999). "Organometallic Fluorides of the Main Group Metals Containing the C—M—F Fragment". in Kenneth D. Karlin. Progress in Inorganic Chemistry. 48 (1 ed.). Wiley. pp. 351–455. doi:10.1002/9780470166499.ch4. ISBN 978-0-471-32623-6. https://onlinelibrary.wiley.com/doi/10.1002/9780470166499.ch4.
- ↑ Ashby, E C.; Yu, Simon H.; Beach, Robert G. (1970). "The Preparation of Alkylmagnesium Fluroindes". Journal of the American Chemical Society 92 (2): 433–435. doi:10.1021/ja00705a634. Bibcode: 1970JAChS..92..433A. https://pubs.acs.org/doi/abs/10.1021/ja00705a634.
- ↑ Ashby, Eugene C.; Yu, Simon H. (1971). "Preparation of alkylmagnesium fluorides". The Journal of Organic Chemistry 36 (15): 2123–2128. doi:10.1021/jo00814a019. https://pubs.acs.org/doi/abs/10.1021/jo00814a019.
- ↑ Peltzer, Raphael M.; Eisenstein, Odile; Nova, Ainara; Cascella, Michele (2017-04-27). "How Solvent Dynamics Controls the Schlenk Equilibrium of Grignard Reagents: A Computational Study of CH3 MgCl in Tetrahydrofuran". The Journal of Physical Chemistry B 121 (16): 4226–4237. doi:10.1021/acs.jpcb.7b02716. PMID 28358509. https://pubs.acs.org/doi/10.1021/acs.jpcb.7b02716.
- ↑ Krasovskiy, Arkady; Knochel, Paul (2006). "Convenient Titration Method for Organometallic Zinc, Harshal ady Magnesium, and Lanthanide Reagents". Synthesis 2006 (5): 890–891. doi:10.1055/s-2006-926345.
- ↑ Henry Gilman and R. H. Kirby (1941). "Butyric acid, α-methyl-". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0361.; Collective Volume, 1, pp. 361
- ↑ Haugan, Jarle André; Songe, Pål; Rømming, Christian; Rise, Frode; Hartshorn, Michael P.; Merchán, Manuela; Robinson, Ward T.; Roos, Björn O. et al. (1997). "Total Synthesis of C31-Methyl Ketone Apocarotenoids 2: The First Total Synthesis of (3R)-Triophaxanthin". Acta Chemica Scandinavica 51: 1096–1103. doi:10.3891/acta.chem.scand.51-1096. http://actachemscand.dk/pdf/acta_vol_51_p1096-1103.pdf. Retrieved 2009-11-26.
- ↑ Agami, Claude; Couty, François; Evano, Gwilherm (2000). "Synthesis of α-Substituted Allylic Amines via a Modified Bruylants Reaction". Organic Letters 2 (14): 2085–2088. doi:10.1021/ol0059908. PMID 10891236.
- ↑ Peters, D. G.; Ji, C. (2006). "A Multistep Synthesis for an Advanced Undergraduate Organic Chemistry Laboratory". Journal of Chemical Education 83 (2): 290. doi:10.1021/ed083p290. Bibcode: 2006JChEd..83..290P.
- ↑ "Unit 12 Aldehydes, Ketones and Carboxylic Acids". Chemistry Part II Textbook for class XII. 2. India: National Council of Educational Research and Training. 2010. pp. 355. ISBN 978-81-7450-716-7. http://ncertbooks.prashanthellina.com/class_12.Chemistry.ChemistryII/Unit%2012.pdf. Retrieved 2019-03-09.
- ↑ Arredondo, Juan D.; Li, Hongmei; Balsells, Jaume (2012). "Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions". Organic Syntheses 89: 460. doi:10.15227/orgsyn.089.0460.
- ↑ A. Fürstner, A. Leitner, G. Seidel (2004). "4-Nonylbenzoic Acid". Organic Syntheses 81: 33–42. http://www.orgsyn.org/demo.aspx?prep=v81p0033.
- ↑ Youhei Nobe; Kyohei Arayama; Hirokazu Urabe (2005). "Air-Assisted Addition of Grignard Reagents to Olefins. A Simple Protocol for a Three-Component Coupling Process Yielding Alcohols". J. Am. Chem. Soc. 127 (51): 18006–18007. doi:10.1021/ja055732b. PMID 16366543. Bibcode: 2005JAChS.12718006N.
- ↑ Benkeser, Robert A. (1971). "The Chemistry of Allyl and Crotyl Grignard Reagents". Synthesis 1971 (7): 347–358. doi:10.1055/s-1971-21738. http://www.thieme-connect.de/DOI/DOI?10.1055/s-1971-21738.
- ↑ Bartolo, Nicole D.; Read, Jacquelyne A.; Valentín, Elizabeth M.; Woerpel, K. A. (2020-02-12). "Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C═N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin–Anh and Chelation-Control Models". Chemical Reviews 120 (3): 1513–1619. doi:10.1021/acs.chemrev.9b00414. PMID 31904936.
- ↑ Richey, Herman Glenn (2000). Grignard Reagents: New Developments. Wiley. ISBN 0471999083.
- ↑ Jordan VC (1993). "Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer". Br J Pharmacol 110 (2): 507–17. doi:10.1111/j.1476-5381.1993.tb13840.x. PMID 8242225.
Further reading
- Rakita, Philip E.; Silverman, Gary S., eds (1996). Handbook of Grignard Reagents. New York, N.Y: Marcel Dekker. ISBN 0-8247-9545-8.
- Mary McHale, "Grignard Reaction," Connexions, http://cnx.org/content/m15245/1.2/. 2007.
- Grignard knowledge: Alkyl coupling chemistry with inexpensive transition metals by Larry J. Westrum, Fine Chemistry November/December 2002, pp. 10–13 [1]
Specialized literature
- Rogers, H. R.; Hill, C. L.; Fujiwara, Y.; Rogers, R. J.; Mitchell, H. L.; Whitesides, G. M. (1980). "Mechanism of formation of Grignard reagents. Kinetics of reaction of alkyl halides in diethyl ether with magnesium". Journal of the American Chemical Society 102 (1): 217. doi:10.1021/ja00521a034. Bibcode: 1980JAChS.102..217R.
- De Boer, H.J.R.; Akkerman, O.S; Bickelhaupt, F. (1988). "Carbanions as intermediates in the synthesis of Grignard Reagents". Angew. Chem. Int. Ed. 27 (5): 687–89. doi:10.1002/anie.198806871.
- Van Klink, G.P.M.; de Boer, H.J.R; Schat, G.; Akkerman, O.S.; Bickelhaupt, F.; Spek, A. (2002). "Carbanions as Intermediates in the Formation of Grignard Reagents". Organometallics 21 (10): 2119–35. doi:10.1021/om011083a.
- Shao, Y.; Liu, Z.; Huang, P.; Liu, B. (2018). "A unified model of Grignard reagent formation". Physical Chemistry Chemical Physics 20 (16): 11100–08. doi:10.1039/c8cp01031e. PMID 29620768. Bibcode: 2018PCCP...2011100S.
 |








