Chemistry:Sarracenin
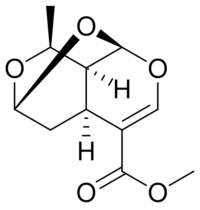
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (1S,3S,7R,8R,9R)-9-methyl-2,4,10-trioxatricyclo[5.3.1.03,8]undec-5-ene-6-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C11H14O5 | |
| Molar mass | 226.228 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sarracenin is an iridoid found in several plant species in the carnivorous family Sarraceniaceae.[1] It also occurs in other non-carnivorous plants such as Strychnos spinosa (Loganiaceae)[2] and Patrinia heterophylla (Caprifoliaceae).[3]
History
Sarracenin was first isolated from the roots of Sarracenia flava in 1976. Analysis of S. flava extracts was prompted by their use as a folk remedy by people of the Okefenokee swamp region[4]
Biosynthesis
Sarracenin is believed to be derived from loganin, with either morronoside or secologanin serving as intermediates in the biosynthetic process.[4]
Uses
Sarracenin displays antimicrobial activity against several pathogens including Staphylococcus aureus, Streptococcus pyogenes, Shigella dysenteriae, Klebsiella pneumonia, Candida albicans, Candida tropicalis, Candida thrusei, and Candida stellatoidea[2]. It has also demonstrated cytotoxicity against three tumor cell lines: A375 (human melanoma cell), SGC-7901 (human gastric cancer cell), and HeLa.[3]
Ecology
Sarracenin is the primary volatile present in the insect-attracting spoons of Heliamphora species. When Heliamphora plants were grown in a laboratory setting, sarracenin was rarely present in these spoons. Plants that did produce sarracenin generally attracted more insects than those without, suggesting a role in prey attraction or capture.[5] Sarracenin is also present in the pitchers and lids of Darlingtonia californica and many Sarracenia species, likely serving the same purpose as in Heliamphora.[1]
References
- ↑ 1.0 1.1 "Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia". PLOS ONE 12 (2): e0171078. 2017-02-21. doi:10.1371/journal.pone.0171078. PMID 28222171. Bibcode: 2017PLoSO..1271078H.
- ↑ 2.0 2.1 "Isolation and Antimicrobial Activity of Sarracenin From Root Bark of Strychnos Spinosa.". Journal of Chemical Society of Nigeria 40 (1). 2015.
- ↑ 3.0 3.1 "Chemical constituents of Patrinia heterophylla Bunge and selective cytotoxicity against six human tumor cells". Journal of Ethnopharmacology 236: 129–135. May 2019. doi:10.1016/j.jep.2019.03.005. PMID 30853646.
- ↑ 4.0 4.1 "Structure of sarracenin. An unusual enol diacetal monoterpene from the insectivorous plant Sarracenia flava". Journal of the American Chemical Society 98 (6): 1569–1573. March 1976. doi:10.1021/ja00422a048.
- ↑ "On insect attractants from pitcher plants of the genusHeliamphora (sarraceniaceae)". Journal of Chemical Ecology 21 (3): 379–384. March 1995. doi:10.1007/BF02036725. PMID 24234068. Bibcode: 1995JCEco..21..379J.
 |

