Chemistry:Screaming jelly babies
File:Screaming gummy bear (chlorate) abridged.ogg
"Screaming Jelly Babies" (British English), also known as "Growling Gummy Bears" (American and Canadian English), is a classroom chemistry demonstration which is practiced in schools around the world.[1] It is often used at open evenings to show the more engaging and entertaining aspects of science in secondary education settings.[2][3]
The experiment shows the amount of energy there is in a piece of candy. Jelly babies[4] or gummy bears[5] are often used for theatrics. Potassium chlorate, a strong oxidising agent, rapidly oxidises the sugar in the candy causing it to burst into flames. The reaction produces a "screaming" sound as rapidly expanding gases are emitted from the test tube.[6] The aroma of caramel is given off.[7] Other carbohydrate or hydrocarbon containing substances can be dropped into test tubes of molten chlorate to produce similar results.[8][9]
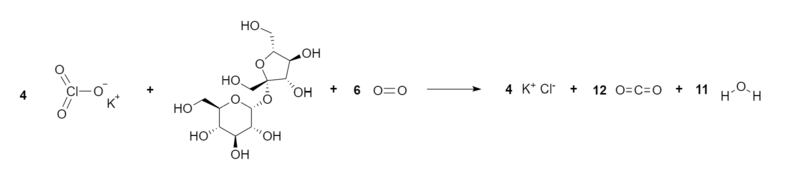
Net Reaction
4 KClO3 (s) + C12H22O11 (s) + 6 O2 (g) → 4 KCl + 12 CO2 (g) + 11 H2O (g)
Mechanism[10]
The solid potassium chlorate is melted into a liquid.
KClO3 (s) + energy → K+ClO3- (l)
The liquid potassium chlorate decomposes into potassium perchlorate and potassium chloride.
4 KClO3 → KCl + 3 KClO4
The potassium perchlorate decomposes into potassium chloride and oxygen.
KClO4 → KCl + 2 O2
The sugar in the candy reacts with oxygen, forming water and carbon dioxide. The reaction is exothermic and produces heat, smoke, and fire.
C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (g) + energy
Safety Measures
Care should be taken in performing this experiment, which should only be attempted by a professional. Potassium chlorate is a strong oxidizer and can cause fire or explosions. It is toxic by inhalation or ingestion and is hazardous to aquatic environments.[11] Reagent grade potassium chlorate should be used. Upon completion of the demonstration, all chemicals should be disposed of in designated chemical waste containers to prevent harm to people or the environment.[7]
All participants in the experiment should wear personal protective equipment, including eye protection, and should stand a safe distance away from the demonstration.[12] A face-shield and heat resistant gloves should be worn by the person adding the jelly baby to the molten potassium chlorate.[12]
Variations
Deviation from the experiment is not recommended, and has been linked with accidents.[12] Candy with low moisture content or high surface area may cause explosions.[12]
References
- ↑ "Growling Gummy Bears". BYU Lecture Prep. Brigham Young University. 22 March 2014. https://secure.chem.byu.edu/lectureprep/node/491.
- ↑ "CHEMISTRY 11 DEMONSTRATIONS". wikieducator.org. 22 July 2011. http://wikieducator.org/images/b/b2/Chem_11_Demos.pdf.
- ↑ "YouTube videos to ignite science". 1 December 2008. http://news.bbc.co.uk/1/hi/education/7758392.stm.
- ↑ "Lubbock Christian University: Can a Gummy Bear Scream?". seatfansclub.com. 22 March 2014. http://www.lcu.edu/about-lcu/professors-with-answers/can-a-gummy-bear-scream.html.
- ↑ "5.5 Oxidation of Sugar or Gummi Bear with Potassium Chlorate". Chemical Reactions II: Oxidation/Reduction. University of Massachusetts Lecture Demonstrations. 22 March 2014. http://lecturedemos.chem.umass.edu/chemReactions5_5.html.
- ↑ "Screaming Jelly Baby Experiment - Student Science". urn1350.net. 16 January 2012. http://www.lcu.edu/about-lcu/professors-with-answers/can-a-gummy-bear-scream.html.
- ↑ 7.0 7.1 "The screaming jelly baby". December 5, 2023. https://www.rsc.org/cpd/teachers/content/filerepository/CMP/00/000/828/CF11_The%20howling%20screaming%20jelly%20baby%20SW.pdf?v=1587952835090.
- ↑ "Jelly Babies". 24 January 2012. https://www.youtube.com/watch?v=mIr4dLGwaVs. Retrieved 18 November 2020.
- ↑ "The University of Nottingham's Periodic Table of Videos". 18 November 2020. https://www.youtube.com/user/periodicvideos. Retrieved 18 November 2020.
- ↑ "Sharing chemistry with the community: "The Exploding Gummy Bear"" (in en). 2017-08-25. https://uwaterloo.ca/chem13-news-magazine/february-2014/activities/sharing-chemistry-community-exploding-gummy-bear.
- ↑ "Potassium Chlorate Safety Data Sheet". December 6, 2023. https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-p/S25482.pdf.
- ↑ 12.0 12.1 12.2 12.3 "Screaming jelly baby – technician notes". January 2021. https://edu.rsc.org/download?ac=509818.
Further reading
- Isherwood, Richard Myers & Bob (2006). World changing ideas. New York: Saatchi & Saatchi. p. 128. ISBN 9780955304606.
- Martin, Jade (November 2, 2011). "Teachers sweeten up chemistry". The Daily Advertiser. http://www.dailyadvertiser.com.au/story/744550/teachers-sweeten-up-chemistry/.
- Maxwell, George (2008). Chemistry Demonstrations For High-School Teachers. Lulu.com. pp. 19–20. ISBN 9780955684302.
- The howling/screaming jelly baby (Report). Royal Society of Chemistry. https://edu.rsc.org/resources/the-howling/screaming-jelly-baby/750.article.
External links
- Jelly Babies - From The University of Nottingham's Periodic Table of Videos
 |