Chemistry:Selenium mustard
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
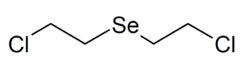
1-Chloro-2-[(2-chloroethyl)selanyl]ethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H8Cl2Se | |
| Molar mass | 205.98 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Selenium mustard (Bis(2-chloroethyl) selenide) is a haloalkyl derivative of selenium, related to vesicant chemical agents such as sulfur mustard and nitrogen mustard. Selenium mustard is somewhat less toxic than its relatives and has not been used as a chemical warfare agent, however it is still a potent alkylating agent and has potential uses in chemotherapy.[1][2][3][4][5]
See also
References
- ↑ "Linear free energy relationships and cytotoxicities of para-substituted 2-haloethyl aryl selenides and bis(2-chloroethyl) selenides". Journal of Medicinal Chemistry 30 (4): 597–602. April 1987. doi:10.1021/jm00387a003. PMID 3560155.
- ↑ "Phenyl selenones: alkyl transfer by selenium-carbon bond cleavage". Journal of Medicinal Chemistry 33 (6): 1544–7. June 1990. doi:10.1021/jm00168a003. PMID 2342050.
- ↑ "Structure-activity studies on organoselenium alkylating agents". Journal of Pharmaceutical Sciences 79 (1): 57–62. January 1990. doi:10.1002/jps.2600790114. PMID 2313578.
- ↑ "One-Pot Two-Step Approach to Selenides. Phase-Transfer Catalyzed Synthesis of ω-Hydroxyalkyl Selenides.". Synthetic Communications 30 (3): 523–529. 2000. doi:10.1080/00397910008087348.
- ↑ "Synthesis of bis(2-haloethyl) selenides by reaction of selenium dihalides with ethylene.". Russian Journal of Organic Chemistry 50 (2): 291–292. February 2014. doi:10.1134/S1070428014020250.
