Chemistry:Sigma complex
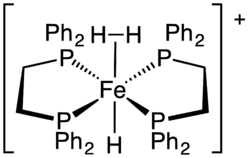
In chemistry, a sigma complex or σ-complex usually refers to a family of coordination complexes where one or more ligands interact with the metal using the bonding electrons in a sigma bond. Transition metal silane complexes are often especially stable sigma complexes. A particularly common subset of sigma complexes are those featuring an agostic interaction where a C–H σ-bond on one of its ligands 'leans' towards and interacts with the coordinatively unsaturated metal center to form a chelate. Transition metal alkane complexes (e.g., a methane complex) that bind solely through the C–H bond are also known but structurally characterized examples are rare, as C–H σ-bonds are generally poor electron donors, and, in many cases, the weakened C–H bond cleaves completely (C–H oxidative addition) to form a complex of type M(R)(H).[1] In some cases, even C–C bonds function as sigma ligands.[2]
Significance
Sigma complexes are of great mechanistic significance, despite their frequent fragility. They represent an initial interaction between the metal center and a hydrocarbon substrate. As such, sigma complexes are generally assumed to be intermediates prior to full oxidative addition.[3]
Types of sigma complexes
Wheland complex
The Wheland complex is an intermediate in the electrophilic substitution reaction on an aromatic compound.[5]
Example - Halogenation of benzene
In the halogenation of benzene, the sigma complex comprises the six carbon atoms of the benzene ring, each bonded to a hydrogen atom. An additional halogen atom is bonded to one of the carbon atoms, which is sp3-hybridized, while the other carbons remain sp2-hybridized. In this state, the ring loses its aromaticity and acquires a positive charge, with the charge delocalized across the ring.[5]
Dihydrogen complexes
Sigma complexes with agostic interactions
Sigma complexes with agostic interactions represent a particularly common subgroup of sigma complexes. In these, a C-H-σ bond from one of the ligands interacts with the coordinatively unsaturated metal center, forming a chelate complex.
Transition metal-alkane complexes
Transition metal-alkane complexes bind exclusively through the C-H bond.
Structurally characterized examples are rare, as C-H σ-bonds generally act as weak electron donors. In many cases, the weakened C-H bond undergoes complete cleavage (oxidative C-H addition).[1]
References
- ↑ 1.0 1.1 Weller, A. S.; Chadwick, F. M.; McKay, A. I. (2016-01-01), Pérez, Pedro J., ed., "Chapter Five - Transition Metal Alkane-Sigma Complexes: Synthesis, Characterization, and Reactivity", Advances in Organometallic Chemistry (Academic Press) 66: pp. 223–276, doi:10.1016/bs.adomc.2016.09.001, https://www.sciencedirect.com/science/article/pii/S0065305516300211, retrieved 2024-08-11
- ↑ Brayshaw, Simon K.; Sceats, Emma L.; Green, Jennifer C.; Weller, Andrew S. (2007-04-24). "C–C σ complexes of rhodium" (in en). Proceedings of the National Academy of Sciences 104 (17): 6921–6926. doi:10.1073/pnas.0609824104. ISSN 0027-8424. PMID 17435164.
- ↑ Kubas, Gregory J. (2001-08-31). Metal Dihydrogen and σ-Bond Complexes: Structure, Theory, and Reactivity. Kluwer. ISBN 0-306-46465-9.
- ↑ Schubert, U.; Scholz, G.; Müller, J.; Ackermann, K.; Wörle, B.; Stansfield, R.F.D. (1986). "Hydrido-silyl-Komplexe". Journal of Organometallic Chemistry 306 (3): 303–326. doi:10.1016/S0022-328X(00)98993-9.
- ↑ 5.0 5.1 "Sigma-Komplex". http://www.u-helmich.de/che/lexikon/S/Sigma-Komplex.html.
 |
