Chemistry:Sporolides
| |
| Names | |
|---|---|
| IUPAC name
(3S,7S,8R,12R,17R,19R,21R,25R)-23-chloro-3,12,17,19,21-pentahydroxy-8-methoxy-5-methyl-2,10,24,26-tetraoxaheptacyclo[11.8.2.27,7.14,18.01,18.016,22.03,25]hexacosa-4,13(23),14,16(22)-tetraene-6,9-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C24H23ClO12 | |
| Molar mass | 538.89 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
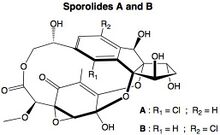
Sporolides A and B are polycyclic macrolides extracted from the obligate marine bacterium Salinispora tropica, which is found in ocean sediment. They are composed of a chlorinated cyclopenta[a]indene ring and a cyclohexenone moiety.[1] They were the second group of compounds (after salinosporamide A) isolated from Salinispora, and were said to indicate the potential of marine actinomycetes as a source of novel secondary metabolites.[2] The structures and absolute stereochemistries of both metabolites were elucidated using a combination of NMR spectroscopy and X-ray crystallography.
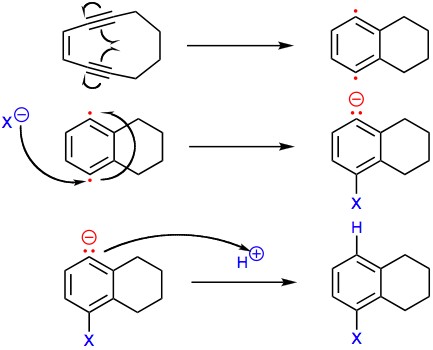
The complex aromatic structure of the sporolides was hypothesized to be derived from an unstable nine-membered ring enediyne precursor, which could undergo Bergman cyclization to generate a para-benzyne intermediate. Nucleophilic attack by chloride would account for the 1:1 mixture of sporolide A and B and for the single chlorine in these enediyne-derived natural products. This proposed mechanism was demonstrated in laboratory experiments,[3]
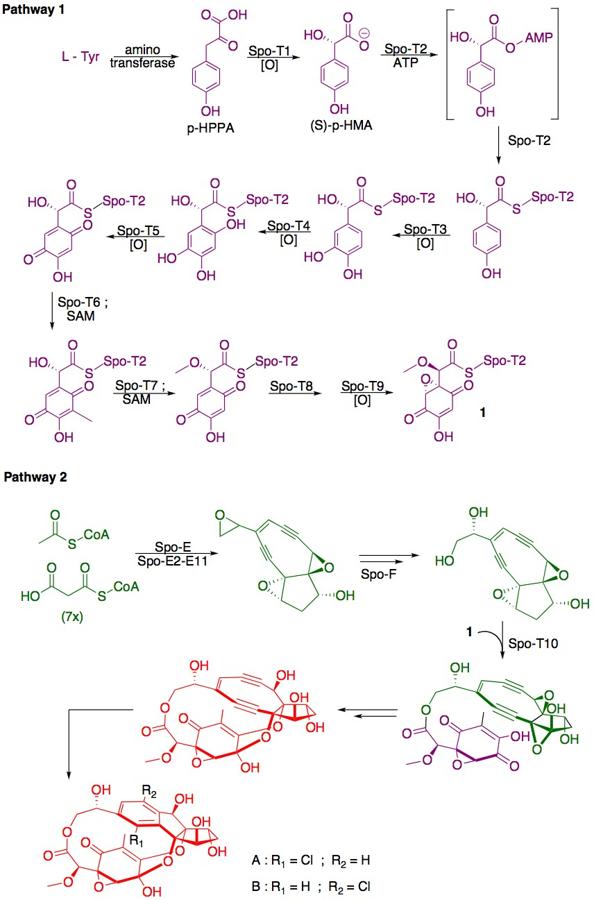
Biosynthesis
The biosynthesis of sporolide A and B is related to that of enediynes such as dynemicin A[4] and is proposed to proceed as shown below.[1][3]
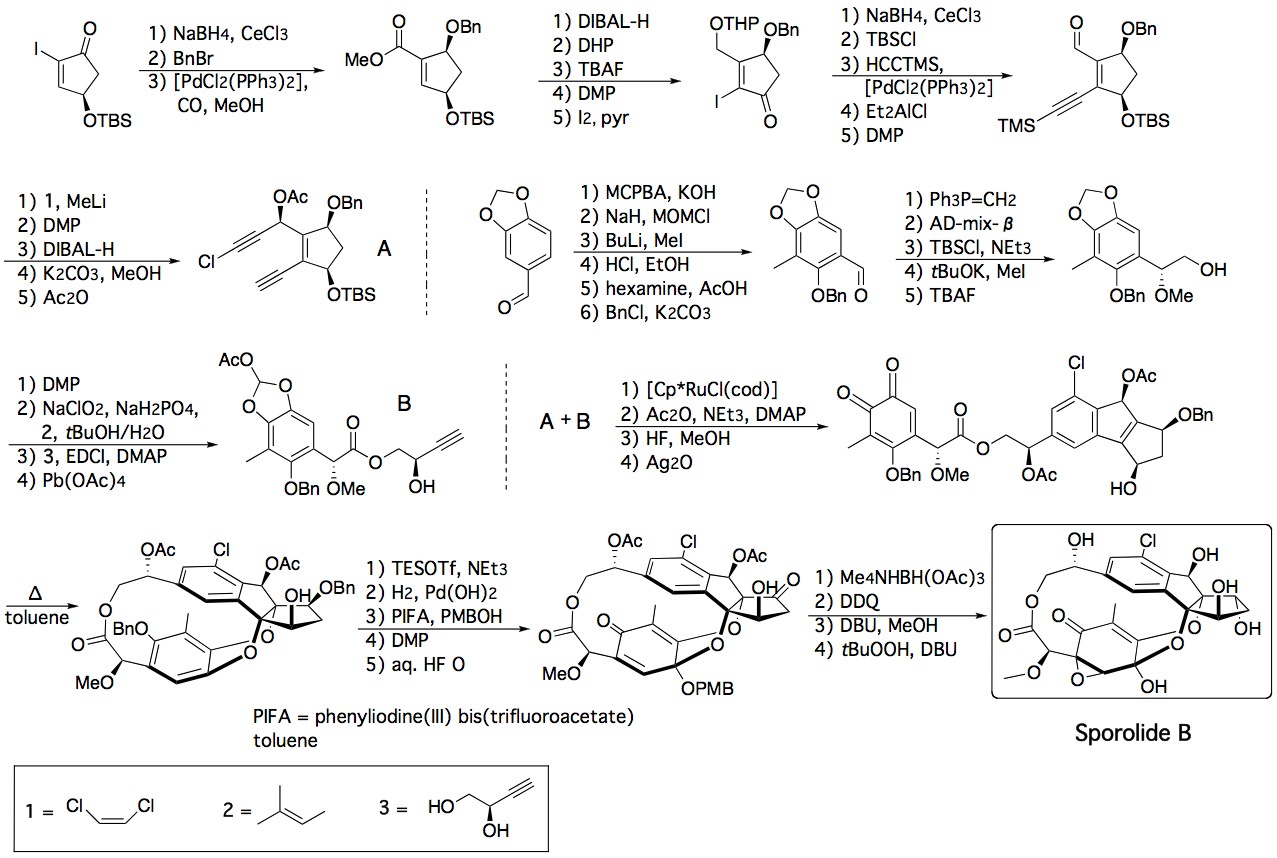
Chemical synthesis
The first total synthesis of sporolide B was reported by K. C. Nicolaou's group and used a highly stereoselective and convergent strategy that involved two cycloaddition reactions. The first was a ruthenium-catalyzed intermolecular [2+2+2] cycloaddition reaction between two acetylenic units, A and B, and the second a thermally induced intramolecular [4+2] cycloaddition reaction between an o-quinone and the tetrasubstituted olefin within the intermediate, forming the macrocyclic structure of the target product.[5][6]
References
- ↑ 1.0 1.1 McGlinchey, Ryan P.; Nett, Markus; Moore, Bradley S. (2008). "Unraveling the Biosynthesis of the Sporolide Cyclohexenone Building Block". Journal of the American Chemical Society 130 (8): 2406–2407. doi:10.1021/ja710488m. PMID 18232689.
- ↑ Buchanan, Greg O.; Williams, Philip G.; Feling, Robert H.; Kauffman, Christopher A.; Jensen, Paul R.; Fenical, William (2005). "Sporolides a and b: Structurally Unprecedented Halogenated Macrolides from the Marine Actinomycete Salinisporatropica". Organic Letters 7 (13): 2731–2734. doi:10.1021/ol050901i. PMID 15957933.
- ↑ 3.0 3.1 Perrin, Charles L.; Rodgers, Betsy L.; O'Connor, Joseph M. (2007). "Nucleophilic Addition to a p-Benzyne Derived from an Enediyne: A New Mechanism for Halide Incorporation into Biomolecules". Journal of the American Chemical Society 129 (15): 4795–4799. doi:10.1021/ja070023e. PMID 17378569.
- ↑ Rudolf, Jeffrey D.; Yan, Xiaohui; Shen, Ben (2016). "Genome neighborhood network reveals insights into enediyne biosynthesis and facilitates prediction and prioritization for discovery". Journal of Industrial Microbiology & Biotechnology 43 (2–3): 261–276. doi:10.1007/s10295-015-1671-0. PMID 26318027.
- ↑ Nicolaou, K. C.; Tang, Yefeng; Wang, Jianhua (2009). "Total Synthesis of Sporolide B". Angewandte Chemie International Edition 48 (19): 3449–3453. doi:10.1002/anie.200900264. PMID 19241430.
- ↑ Nicolaou, K. C.; Wang, Jianhua; Tang, Yefeng; Botta, Lorenzo (2010). "Total Synthesis of Sporolide B and 9-epi-Sporolide B". Journal of the American Chemical Society 132 (32): 11350–11363. doi:10.1021/ja1048994. PMID 20698702.
 |

