Chemistry:Subhalide
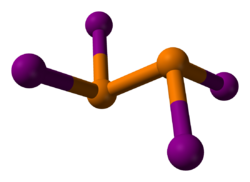
2I
4 is a subiodide of phosphorus.
In chemistry, subhalide usually refers to inorganic compounds that have a low ratio of halide to metal, made possible by metal–metal bonding (or element–element bonding for nonmetals), sometimes extensive. Many compounds meet this definition.[citation needed]
Examples
The normal halide of boron is BF
3. Boron forms many subhalides: several B
2X
4, including B
2F
4; also BF. Aluminium forms a variety of subhalides. For gallium, adducts of Ga
2Cl
4 are known. Phosphorus subhalides include P
2I
4, P
4Cl
2, and P
7Cl
3 (structurally related to [P
7]3−). For bismuth, the compound originally described as bismuth monochloride was later shown to consist of [Bi
9]5+ clusters and chloride anions.[1] There are many tellurium subhalides, including Te
3Cl
2, Te
2X (X = Cl, Br, I), and two forms of TeI.[2]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Zhengtao Xu "Recent Developments in Binary Halogen–Chalcogen Compounds, Polyanions and Polycations" in Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, Francesco Devillanova, Editor, 2006, RSC. pp. 381-416. Royal Society doi:10.1039/9781847557575-00455
 |
