Chemistry:Diphosphorus tetraiodide
| |
| Names | |
|---|---|
| IUPAC name
Diphosphorus tetraiodide
| |
| Preferred IUPAC name
Tetraiododiphosphane | |
| Other names
Phosphorus(II) iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| P2I4 | |
| Molar mass | 569.57 g/mol |
| Appearance | Orange crystalline solid |
| Melting point | 125.5 °C (257.9 °F; 398.6 K) |
| Boiling point | Decomposes |
| Decomposes | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Diphosphorus tetrafluoride Diphosphorus tetrachloride Diphosphorus tetrabromide |
Other cations
|
diarsenic tetraiodide |
Related Binary Phosphorus halides
|
phosphorus triiodide |
Related compounds
|
diphosphane diphosphines |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
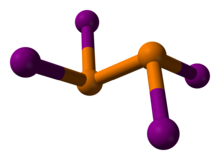
Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.[1]
Synthesis and structure
Diphosphorus tetraiodide is easily generated by the disproportionation of phosphorus triiodide in dry ether:
- 2 PI
3 → P
2I
4 + I
2
It can also be obtained by treating phosphorus trichloride and potassium iodide in anhydrous conditions.[2]
Another synthesis route involves combining phosphonium iodide with iodine in a solution of carbon disulfide. An advantage of this route is that the resulting product is virtually free of impurities.[3]
- 2PH
4I + 5I
2 → P
2I
4 + 8HI
The compound adopts a centrosymmetric structure with a P-P bond of 2.230 Å.[4]
Reactions
Inorganic chemistry
Diphosphorus tetraiodide reacts with bromine to form mixtures PI3−xBrx. With sulfur, it is oxidized to P2S2I4, retaining the P-P bond.[1] It reacts with elemental phosphorus and water to make phosphonium iodide, which is collected via sublimation at 80 °C.[3]
Organic chemistry
Diphosphorus tetraiodide is used in organic synthesis mainly as a deoxygenating agent.[5] It is used for deprotecting acetals and ketals to aldehydes and ketones, and for converting epoxides into alkenes and aldoximes into nitriles. It can also cyclize 2-aminoalcohols to aziridines[6] and to convert α,β-unsaturated carboxylic acids to α,β-unsaturated bromides.[7]
As foreshadowed by the work of Bertholet in 1855, diphosphorus tetraiodide can convert glycols to trans alkenes.[5][8] This reaction is known as the Kuhn–Winterstein reaction, after the chemists who applied it to the production of polyene chromophores.[5][9]
References
- ↑ 1.0 1.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ H. Suzuki; T. Fuchita; A. Iwasa; T. Mishina (December 1978). "Diphosphorus Tetraiodide as a Reagent for Converting Epoxides into Olefins, and Aldoximes into Nitriles under Mild Conditions". Synthesis 1978 (12): 905–908. doi:10.1055/s-1978-24936.
- ↑ 3.0 3.1 Brown, Glenn Halstead (1951). Reactions of phosphine and phosphonium iodide (PhD). Iowa State College. Retrieved 5 Oct 2020.
- ↑ Z. Žák; M. Černík (1996). "Diphosphorus tetraiodide at 120 K". Acta Crystallographica Section C C52 (2): 290–291. doi:10.1107/S0108270195012510.
- ↑ 5.0 5.1 5.2 Krief, Alain; Telvekar, Vikas N. (2009). "Diphosphorus Tetraiodide". Encyclopedia for Reagents in Organic Synthesis 2009. doi:10.1002/047084289X.rd448.pub2. ISBN 978-0471936237.
- ↑ H. Suzuki; H. Tani (1984). "A mild cyclization of 2-aminoalcohols to aziridines using diphosphorus tetraiodide". Chemistry Letters 13 (12): 2129–2130. doi:10.1246/cl.1984.2129.
- ↑ Vikas N. Telvekar; Somsundaram N. Chettiar (June 2007). "A novel system for decarboxylative bromination". Tetrahedron Letters 48 (26): 4529–4532. doi:10.1016/j.tetlet.2007.04.137.
- ↑ Kuhn, Richard; Winterstein, Alfred (1928). "Über konjugierte Doppelbindungen I. Synthese von Diphenyl-poly-enen" (in de). Helvetica Chimica Acta 11 (1): 87–116. doi:10.1002/hlca.19280110107.
- ↑ Inhoffen, H. H.; Radscheit, K.; Stache, U.; Koppe, V. (1965). "Untersuchungen an hochsubstituierten äthylenen und Glykolen, II. Synthese des 3.4-Bis-[4-oxo-cyclohexyl]-hexens-(3) mit Hilfe der Kuhn-Winterstein-Reaktion" (in de). Justus Liebigs Ann. Chem. (684): 24–36. doi:10.1002/jlac.19656840106.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

