Chemistry:Tartronic acid semialdehyde
From HandWiki
| |
| Names | |
|---|---|
| Other names
Tartronaldehydic acid
2-hydroxy-3-oxopropanoic acid formyl(hydroxy)acetic acid Hydroxymalonaldehydic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
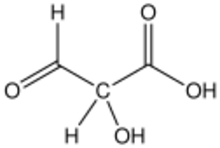
| C3H4O4 | |
| Molar mass | 104.061 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tartronic acid semialdehyde is the organic compound with the formula OCHCH(OH)CO2H. The molecule has three functional groups, aldehyde, alcohol, and carboxylic acid. A white solid, it occurs naturally. A near neutral pH, it exists as the hydrated carboxylate (HO)2CHCH(OH)CO2−, which is referred to as tartronate semialdehyde. Tartronate semialdehyde is produced and consumed on a prodigious scale as an intermediate in photorespiration, an undesirable side reaction that competes with photosynthesis. It is produced biologically by the condensation of two equivalents of glyoxalate:[1]
- 2 OC(H)CO2H → OC(H)CH(OH)CO2H + CO2
This condensation is catalyzed by tartronate-semialdehyde synthase.
References
- ↑ "A Welcome Diversion from Photorespiration". Nature Biotechnology 25 (5): 539–40. May 2007. doi:10.1038/nbt0507-539. PMID 17483837.
 |

