Chemistry:Terpestacin
From HandWiki
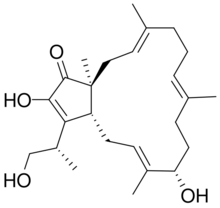
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,5E,7S,10E,14E,16aS)-2,7-Dihydroxy-3-[(2S)-1-hydroxypropan-2-yl]-6,10,14,16a-tetramethyl-4,7,8,9,12,13,16,16a-octahydrocyclopenta[15]annulen-1(3aH)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H38O4 | |
| Molar mass | 402.575 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Terpestacin[1] is a fungal metabolite with anticancer activity.
References
- ↑ Chan, Johann; Jamison, Timothy F. (September 2003). "Synthesis of (−)-Terpestacin via Catalytic, Stereoselective Fragment Coupling: Siccanol Is Terpestacin, Not 11- -Terpestacin". Journal of the American Chemical Society 125 (38): 11514–11515. doi:10.1021/ja0373925. PMID 13129351. https://figshare.com/articles/journal_contribution/3655356/files/5744838.pdf.
External links
- "Alteration of membrane polarization by (−)-terpestacin, a biologically active fungal metabolite". Die Pharmazie 57 (9): 653–4. September 2002. PMID 12369460.
- "Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing". J Biol Chem 285 (15): 11584–95. April 2010. doi:10.1074/jbc.M109.087809. PMID 20145250.
- "Effects of endostatin and a new drug terpestacin against human neuroblastoma xenograft and cell lines". Pediatric Surgery International 29 (12): 1327–40. December 2013. doi:10.1007/s00383-013-3398-1. PMID 24072200.
 |

