Chemistry:Tetrahydropyridine
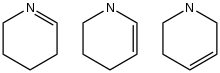 Three isomers of tetrahydropyridine
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
| UN number | 2410 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9N | |
| Molar mass | 83.134 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrahydropyridines (or piperideines) are heterocycles with the formula C
5H
9N. Three isomers exist, which differ by the location of the double bond. All three are chiral. None of the parent species occur widely, so they are mainly of theoretical interest. Although the parent tetrahydropyridines are rare, many substituted tetrahydropyridines are known.
Preparation and occurrence

1,2,3,6-Tetrahydropyridine, a colorless liquid, is commercially available. It is an imine.
Illustrating another isomer of tetrahydropyridine, 6-acetyl-2,3,4,5-tetrahydropyridine occurs naturally.
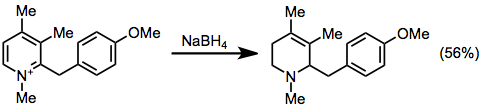
Partial reduction of pyridinium salts gives N-alkyltetrahydropyridines. Treatment of N-methylpyridinium with borohydride reagents gives 1-methyl-1,2,3,6-tetrahydropyridine.[1][2]
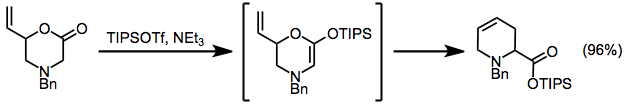
A modified Ireland-Claisen rearrangement leads to tetrahydropyridines via a silyl ketene acetal intermediate.[3]
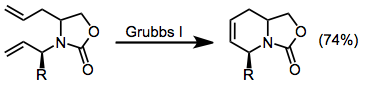
Ring-closing olefin metathesis has also been used to establish the tetrahydropyridine ring system.[4]
References
- ↑ Balasubramanian, Marudai (2013). "Formation of Completely or Partially Reduced Pyridines and Quinolines". Pyridines: From lab to production. pp. 413–458. doi:10.1016/B978-0-12-385235-9.00005-9. ISBN 9780123852359.
- ↑ Thyagarajan, G.; May, E. L. (1971). "Improved synthesis of 2-benzyl-1,2,5,6-tetrahydropyridines, precursors of analgetic 6,7-benzomorphans". J. Heterocycl. Chem. 8 (3): 465. doi:10.1002/jhet.5570080317.
- ↑ Angle, S. R.; Henry, R. M. (1998). "Studies toward the Synthesis of (+)-Palustrine: The First Asymmetric Synthesis of (−)-Methyl Palustramate". J. Org. Chem. 63 (21): 7490–7497. doi:10.1021/jo980749g. PMID 11672402.
- ↑ Deiters, A.; Martin, S. F. (2004). "Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis". Chem. Rev. 104 (5): 2199–238. doi:10.1021/cr0200872. PMID 15137789.
 |