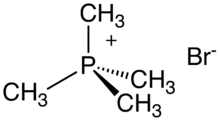
Chemistry:Tetramethylphosphonium bromide
From HandWiki
Short description: A white, water-soluble organophosphorus compound
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H12BrP | |
| Molar mass | 171.018 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetramethylphosphonium bromide is an organophosphorus compound with the formula (CH3)4PBr. It is a white, water-soluble solid, the salt of the cation tetramethylphosphonium and the bromide anion. It is prepared by treating trimethylphosphine with methyl bromide.
Reactions
Deprotonation gives methylenetrimethylphosphine ylide, which can sustain a second deprotonation:[1]
- (CH3)4PBr + BuLi → CH3)3P=CH2 + LiBr + BuH
- CH3)3P=CH2 + BuLi → CH3)2P(CH2)2Li + BuH
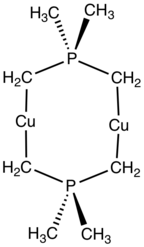
The latter is a precursor to many coordination complexes, e.g., the dicuprous complex Cu2[(Me2P(CH2)2]2.[2]
References
- ↑ H. F. Klein (1978). "Trimethylphosphonium Methylide (Trimethyl Methylenephosphorane)". Inorganic Syntheses XVIII: 138-140. doi:10.1002/9780470132494.ch23.
- ↑ Schmidbaur, H. (1983). "Phosphorus Ylides in the Coordination Sphere of Transition Metals: An Inventory". Angewandte Chemie International Edition in English 22 (12): 907–927. doi:10.1002/anie.198309071.
 |