Chemistry:Thulium(III) selenate
From HandWiki
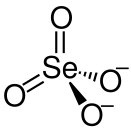
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| O12Se3Tm2 | |
| Molar mass | 766.769 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thulium(III) selenate is an inorganic compound, with the chemical formula Tm2(SeO4)3. It can be obtained by reacting a thulium(III) oxide and selenic acid solution and crystallizing it.[1] It crystallises with ammonium selenate in an aqueous solution to obtain NH4Tm(SeO4)2·3H2O.[2]
Thulium(III) selenate decomposes at high temperatures and passes through Tm2(SeO3)3 to finally obtain the oxide Tm2O3.[3]
References
- ↑ Wickleder, Mathias S. (2005), Oxo-Selenates of rare earth elements, Handbook on the Physics and Chemistry of Rare Earths, 35, Elsevier, pp. 45–105, doi:10.1016/s0168-1273(05)35002-1, ISBN 9780444520289, http://dx.doi.org/10.1016/s0168-1273(05)35002-1, retrieved 2023-11-29
- ↑ "Zhurnal Neorganicheskoi Khimii (Inorganic Chemistry) is 50". Russian Journal of Inorganic Chemistry 51 (5): 844–845. May 2006. doi:10.1134/s0036023606050287. ISSN 0036-0236. http://dx.doi.org/10.1134/s0036023606050287.
- ↑ Bohumil Hajek, Nadezda Novotna, Jarmila Hradilova (Aug 1979). "Studies of thermal decompositions and infrared spectra of the rare earth selenate octahydrates Ln2(SeO4)3· 8H2O (Ln = Y,Tb,Dy,Ho,Er,Tm,Yb,Lu)" (in en). Journal of the Less Common Metals 66 (2): 121–136. doi:10.1016/0022-5088(79)90222-4. https://linkinghub.elsevier.com/retrieve/pii/0022508879902224. Retrieved 2020-05-29.
 |

