Chemistry:Toluidine red
From HandWiki
| |
| Names | |
|---|---|
| Other names
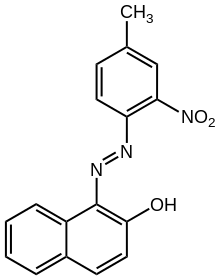
Pigment Red 3, 1-(4-Methyl-2-nitrophenylazo)-2-naphthol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H13N3O3 | |
| Molar mass | 307.309 g·mol−1 |
| Appearance | red solid |
| Density | 1.434 g/cm3[1] |
| low | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H318, H410, H413 | |
| P264+265Script error: No such module "Preview warning".Category:GHS errors, P273, P280, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Toluidine red is an organic compound with the formula C
10H
6(OH)(N
2C
6H
3(NO
2)CH
3). A dark red solid, the compound is classified as a azo dye consisting of a 2-naphthol group linked to a 2-nitro-4-methylphenyl substituent.[3] Toluidine red is a traditional pigment, found in oil paints.[4] Although once popular, it suffers as a pigment owing to "insufficient lightfastness and bleeding when incorporated into a paint system."[1]
Safety
It is classified as carcinogenic, a property that it shares with many azo dyes.[5]
References
- ↑ 1.0 1.1 Chung, F. H. (1971). "Crystallography of Toluidine Red". Journal of Applied Crystallography 4 (1): 79–80. doi:10.1107/S0021889871006307. Bibcode: 1971JApCr...4...79C.
- ↑ "Toluidine red" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/17047#section=Safety-and-Hazards.
- ↑ Jaffe, Edward E. (2004). "Pigments, Organic". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.151807011001060605.a01.pub2. ISBN 978-0-471-48494-3.
- ↑ Scherrer, Nadim C.; Stefan, Zumbuehl; Francoise, Delavy; Annette, Fritsch; Renate, Kuehnen (2009). "Synthetic Organic Pigments of the 20th and 21st Century Relevant to Artist's Paints: Raman Spectra Reference Collection". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 73 (3): 505–524. doi:10.1016/j.saa.2008.11.029. PMID 19136293. Bibcode: 2009AcSpA..73..505S.
- ↑ Møller, Peter; Wallin, Håkan (2000). "Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene". Mutation Research/Reviews in Mutation Research 462 (1): 13–30. doi:10.1016/s1383-5742(99)00090-3. PMID 10648921.
 |

