Chemistry:Triafulvalene
From HandWiki
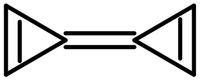
| |
| Names | |
|---|---|
| Preferred IUPAC name
[1,1′-Bi(cyclopropylidene)]-2,2′-diene | |
| Other names
1,1′-Bi(cycloprop-2-en-1-ylidene)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H4 | |
| Molar mass | 76.098 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Triafulvalene or cyclopropenylidenecyclopropene is a fulvalene hydrocarbon with chemical formula C6H4, composed of two linked cyclopropene rings. Triafulvalene has never been isolated,[1] since it can decompose via an isodesmic reaction.[2] However, this molecule is of theoretical significance for theoretical organic chemists,[3][why?] and its structure, stability, and spectral properties are well-studied.[citation needed]
See also
References
- ↑ Carey, Francis A.; Richard J. Sundberg (2007). Advanced Organic Chemistry: Part A: Structure and Mechanisms. Springer Science & Business Media. pp. 755–787. ISBN 978-0-387448-99-2. https://books.google.com/books?id=etMn6bCjaE0C&q=possibility+dipolar+resonance+structures&pg=PA754.
- ↑ Neuenschwander, Markus (1986), "Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes", Pure Appl. Chem. 58 (1): 55–66, doi:10.1351/pac198658010055, http://pac.iupac.org/publications/pac/pdf/1986/pdf/5801x0055.pdf
- ↑ Scott, Anthony P.; Agranat, Israel; Biedermann, P. Ulrich; Riggs, Noel V.; Radom, Leo (1997). "Fulvalenes, Fulvenes, and Related Molecules: An ab Initio Study" (in en). The Journal of Organic Chemistry 62 (7): 2026–2038. doi:10.1021/jo962407l. ISSN 0022-3263. PMID 11671506. https://pubs.acs.org/doi/10.1021/jo962407l.
 |

