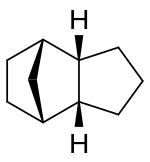
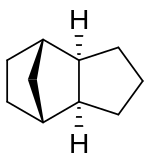
Chemistry:Tricyclodecane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tricyclo[5.2.1.02,6]decane
| |||
| Other names
Tetrahydrodicyclopentadiene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Hazards | |||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H226, H302, H304, H315, H319, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P301+316Script error: No such module "Preview warning".Category:GHS errors, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P303+361+353, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P331, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors | |||
| Related compounds | |||
Related compounds
|
Twistane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tricyclodecane (TCD) is an organic compound with the formula C10H16. It is classed as a hydrocarbon. It has two main stereoisomers–the endo and exo forms.[2] Its primary use in the exo form is as a component of jet fuel.[3] It is used here primarily because of its high energy density. The exo isomer also has a low freezing point.[4][5] Because of this, its properties have been studied extensively.[6][7][8][9][10] It is often called tetrahydrodicyclopentadiene.
Reactions
Its reactions with other materials has been studied,[11][12] as have various production methods.[13][14] The two isomers can interconvert in the presence of aluminum chloride as catalyst absorbed on substrates such as silicon dioxide or zeolites,[15][16][17][18] with preference for forming the exo as the major product.[19][20]
References
- ↑ "Tetrahydrodicyclopentadiene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/17795#section=Safety-and-Hazards.
- ↑ "Exo-tricyclo[5.2.1.0(2.6)decane"] (in en). https://webbook.nist.gov/cgi/cbook.cgi?ID=U215287&Units=SI&Mask=80.
- ↑ Hudzik, Jason M.; Asatryan, Rubik; Bozzelli, Joseph W. (2010-09-09). "Thermochemical Properties of exo -Tricyclo[5.2.1.0 2,6 decane (JP-10 Jet Fuel) and Derived Tricyclodecyl Radicals"] (in en). The Journal of Physical Chemistry A 114 (35): 9545–9553. doi:10.1021/jp1049556. ISSN 1089-5639. PMID 20712369. Bibcode: 2010JPCA..114.9545H. https://pubs.acs.org/doi/10.1021/jp1049556.
- ↑ Herbinet, Olivier; Sirjean, Baptiste; Bounaceur, Roda; Fournet, René; Battin-Leclerc, Frédérique; Scacchi, Gérard; Marquaire, Paul-Marie (2006-10-01). "Primary Mechanism of the Thermal Decomposition of Tricyclodecane" (in en). The Journal of Physical Chemistry A 110 (39): 11298–11314. doi:10.1021/jp0623802. ISSN 1089-5639. PMID 17004739. Bibcode: 2006JPCA..11011298H. https://pubs.acs.org/doi/10.1021/jp0623802.
- ↑ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame 216: 82–91. doi:10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. https://www.sciencedirect.com/science/article/pii/S0010218020300821.
- ↑ "Exo-tricyclo[5.2.1.0(2.6)decane"] (in en). https://www.chemeo.com/cid/70-324-4/Exo-tricyclo-5-2-1-0-2-6-decane.
- ↑ Seiser, R.; Niemann, U.; Seshadri, K. (2011-01-01). "Experimental study of combustion of n-decane and JP-10 in non-premixed flows". Proceedings of the Combustion Institute 33 (1): 1045–1052. doi:10.1016/j.proci.2010.06.078. ISSN 1540-7489. https://www.sciencedirect.com/science/article/pii/S1540748910001380.
- ↑ Tao, Yujie; Xu, Rui; Wang, Kun; Shao, Jiankun; Johnson, Sarah E.; Movaghar, Ashkan; Han, Xu; Park, Ji-Woong et al. (2018-12-01). "A Physics based approach to modeling real fuel combustion chemistry III Reaction kinetic model of JP10". Combustion and Flame 198: 466–476. doi:10.1016/j.combustflame.2018.08.022. ISSN 0010-2180. https://www.sciencedirect.com/science/article/pii/S0010218018303870.
- ↑ Li, Heng; Liu, Guozhu; Jiang, Rongpei; Wang, Li; Zhang, Xiangwen (2015-05-01). "Experimental and kinetic modeling study of exo-TCD pyrolysis under low pressure". Combustion and Flame 162 (5): 2177–2190. doi:10.1016/j.combustflame.2015.01.015. ISSN 0010-2180. https://www.sciencedirect.com/science/article/pii/S0010218015000188.
- ↑ Goh, K. H. H.; Geipel, P.; Hampp, F.; Lindstedt, R. P. (2013-01-01). "Regime transition from premixed to flameless oxidation in turbulent JP-10 flames". Proceedings of the Combustion Institute 34 (2): 3311–3318. doi:10.1016/j.proci.2012.06.173. ISSN 1540-7489. https://www.sciencedirect.com/science/article/pii/S1540748912002817.
- ↑ "STTR Navy FY09A - Characterization of the High Temperature Decomposition Products of JP-10". https://www.navysbir.com/n09_s/navst09-011.htm.
- ↑ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame 216: 82–91. doi:10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. https://www.sciencedirect.com/science/article/pii/S0010218020300821.
- ↑ Büchner, Karl; Otto Roelen & Josef Meis, "Production of tricyclodecane", US patent 2766301, published 1956-10-09, assigned to Ruhrchemie AG
- ↑ Baptiste, Sirjean. "Theoretical Study of the Thermal Decomposition of a Jet Fuel Surrogate". https://hal.science/hal-00377675/document.
- ↑ Sun, Cong-ming; Li, Gang (2011-07-31). "Vapor-phase isomerization of endo-tetrahydrodicyclopentadiene to its exo isomer over zeolite catalysts". Applied Catalysis A: General 402 (1): 196–200. doi:10.1016/j.apcata.2011.06.008. ISSN 0926-860X. https://www.sciencedirect.com/science/article/pii/S0926860X11003437.
- ↑ Campo, Pablo del; Martínez, Cristina; Corma, Avelino (2021-08-02). "Activation and conversion of alkanes in the confined space of zeolite-type materials" (in en). Chemical Society Reviews 50 (15): 8511–8595. doi:10.1039/D0CS01459A. ISSN 1460-4744. PMID 34128513. https://pubs.rsc.org/en/content/articlelanding/2021/cs/d0cs01459a.
- ↑ Navrátilová, Markéta; Sporka, Karel (2000-09-18). "Synthesis of adamantane on commercially available zeolitic catalysts". Applied Catalysis A: General 203 (1): 127–132. doi:10.1016/S0926-860X(00)00477-4. ISSN 0926-860X. https://www.sciencedirect.com/science/article/pii/S0926860X00004774.
- ↑ Gallego, Eva María; Portilla, M. Teresa; Paris, Cecilia; León-Escamilla, Alejandro; Boronat, Mercedes; Moliner, Manuel; Corma, Avelino (2017-03-10). ""Ab initio" synthesis of zeolites for preestablished catalytic reactions" (in en). Science 355 (6329): 1051–1054. doi:10.1126/science.aal0121. ISSN 0036-8075. PMID 28280200. Bibcode: 2017Sci...355.1051G. https://www.science.org/doi/10.1126/science.aal0121.
- ↑ Lili, Q. I.; Min*, J. I.; Xinkui, Wang; Min, H. E.; Tianxi, C. a. I. (2010-04-25). "AlCl3/MCM-41 as a Catalyst for Isomerization of Endo-tricyclodecane" (in en). Chinese Journal of Catalysis 31 (4): 383. ISSN 0253-9837. https://www.cjcatal.com/EN/.
- ↑ Clarke, J. K. A.; Rooney, J. J. (1976-01-01), Eley, D. D.; Pines, Herman; Weisz, Paul B., eds., Stereochemical Approaches to Mechanisms of Hydrocarbon Reactions on Metal Catalysts, Advances in Catalysis, 25, Academic Press, pp. 125–183, doi:10.1016/s0360-0564(08)60314-4, ISBN 9780120078257, https://www.sciencedirect.com/science/article/pii/S0360056408603144, retrieved 2023-11-20
 |