Chemistry:Trioctylmethylammonium bis(trifluoromethylsulfonyl)imide
From HandWiki
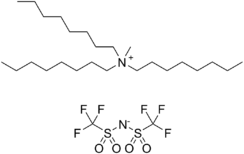
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-methyl-N,N-di(octyl)octan-1-aminium bis(trifluoromethanesulfonyl)azanide | |
| Other names
Methyl-trioctylammonium bis(trifluoromethylsulfonyl)imide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H54F6N2O4S2 | |
| Molar mass | 648.85 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P337+313, P362, P403+233, P405 | |
| Flash point | >110 °C |
| Related compounds | |
Related compounds
|
Bistriflimide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Trioctylmethylammonium bis(trifluoromethylsulfonyl)imide is an ionic liquid[3] that is produced by Solvent Innovation, now part of EMD Chemicals.
References
- ↑ See PubChem
- ↑ "Methyltrioctylammonium bis(trifluoromethylsulfonyl)imide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/16211009#section=Safety-and-Hazards.
- ↑ Fraile, J. M.; Garcia, J. I.; Herrerias, C. I.; Mayoral, J. A.; Carrie, D.; Vaultier, M. (2001). "Enantioselective cyclopropanation reactions in ionic liquids". Tetrahedron: Asymmetry 12 (13): 1891–1894. doi:10.1016/s0957-4166(01)00315-9.
 |

