Chemistry:Tris(bipyridine)iron(II) chloride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C30H24Cl2FeN6 | |
| Molar mass | 595.31 g·mol−1 |
| Appearance | red solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
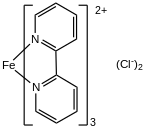
Tris(bipyridine)iron(II) chloride is the chloride salt of the coordination complex tris(bipyridine)iron(II), [Fe(C
10H
8N
2)
3]2+. It is a red solid. In contrast to tris(bipyridine)ruthenium(II), this iron complex is not a useful photosensitizer because its excited states relax too rapidly, a consequence of the primogenic effect.
Tris(bipyridine)iron(II) chloride features an octahedral Fe(II) center bound to three 2,2'-Bipyridine ligands. The complex has been isolated as salts with many anions. [1]
Synthesis and reactions
The sulfate salt [Fe(bipy)
3]SO
4 is produced by combining ferrous sulfate with excess bipy in aqueous solution. This result illustrates the preference of Fe(II) for bipyridine vs water. Addition of cyanide to this solution precipitates solid Fe(bipy)
2(CN)
2.[2]
Related complexes
Reference
- ↑ Batten, Stuart R.; Murray, Keith S.; Sinclair, Nathan J. (2000). "Tris(2,2′-bipyridyl- N , N ′)iron(II) diperchlorate". Acta Crystallographica Section C Crystal Structure Communications 56 (8): e320. doi:10.1107/S0108270100009185.
- ↑ Schilt, Alfred A. (1970). "Dicyanobis(1,10‐phenanthroline)Iron(II) and Dicyanobis(2,2′‐bipyridine)iron(II)". Inorganic Syntheses 12: 247–251. doi:10.1002/9780470132432.ch43. ISBN 9780470131718.
 |

