Chemistry:Utopioid (drug class)
Utopioids (U-type opioids) are a class of synthetic opioid analgesic drugs first developed in the 1970s by the pharmaceutical company Upjohn.[1] However, they were never marketed for medical use. Some compounds from this class have been used for scientific research as model kappa opioid receptor agonists. In the mid-2010s, one mu opioid receptor selective compound from this class, U-47700, re-emerged as a designer drug and became widely sold around the world for several years before being banned in various jurisdictions from 2016 onwards. Following the prohibition of U-47700, a number of related compounds have continued to appear on illicit drug markets. They are often marketed online or included as components in mixtures sold under the guise of "street heroin." U-47700 itself is the most potent mu opioid agonist from this class, around 7-10x the potency of morphine. Some other compounds such as 3,4-MDO-U-47700 and N-Ethyl-U-47700 retain similar mu selectivity but with lower potency similar to that of morphine, or have a mixture of mu and kappa mediated effects, such as U-48800. Most utopioid derivatives are however selective kappa agonists, which may have limited abuse potential as dissociative hallucinogens, but do not alleviate withdrawal distress in opioid dependent individuals or maintain addiction in a typical sense. Nevertheless, this has not stopped them from being sold as designer drugs, and a number of these compounds are now banned in many jurisdictions alongside U-47700 itself.[2][3][4][5][6][7][8][9]
Table of Utopioids
| Chemical structure | Drug name | PubChem | CAS number |
|---|---|---|---|
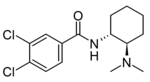
|
U-47109 | 44269286 | 67579-13-9 |
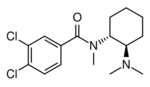
|
U-47700 | 13544016 | 82657-23-6 |
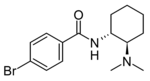
|
U-47931E (Bromadoline) | 6328449 | 2418521-61-4 |
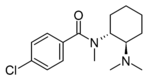
|
U-48520 | 13544026 | 67579-11-7 |
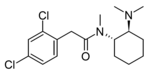
|
U-48800 | 137700072 | 2370977-17-4 |
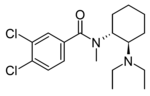
|
U-49900 | 129392412 | 67579-76-4 |
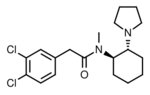
|
U-50488 | 3036289 | 67198-13-4 |
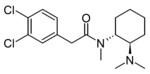
|
U-51574 | 44269303 | |
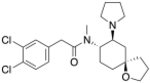
|
U-62066 (Spiradoline) | 55652 | 87151-85-7 |
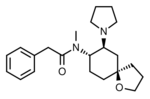
|
U-69593 | 105104 | 96744-75-1 |
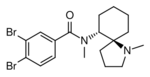
|
U-77891 | 117071705 | 119878-31-8 |
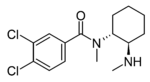
|
N-Desmethyl-U-47700 | 129390993 | 67579-73-1 |
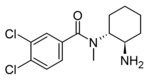
|
N,N-Didesmethyl-U-47700 | 129406364 | 2616858-81-0 |
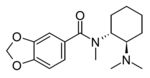
|
3,4-MDO-U-47700 | 139598237 | 2488874-96-8 |
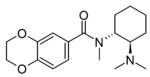
|
3,4-Ethylenedioxy-U-47700 | 137700298 | 2749619-08-5 |
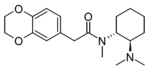
|
3,4-Ethylenedioxy-U-51574 | 137700374 | 2748623-91-6 |
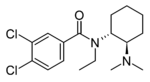
|
N-Ethyl-U-47700 | 155907846 | |
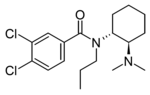
|
N-Propyl-U-47700 | 137700434 | 2749433-76-7 |
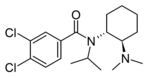
|
N-Isopropyl-U-47700 | 137700166 | 2748319-16-4 |
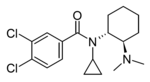
|
N-Cyclopropyl-U-47700 | 165361451 | |
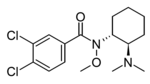
|
N-Methoxy-U-47700 | 155907659 | |
|
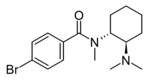
N-Methyl-U-47931E | 54482637 | 75570-38-6 |
|
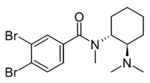
3,4-Dibromo-U-47700 | ||
|
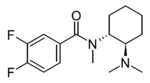
3,4-Difluoro-U-47700 | 165362347 | 2417942-54-0 |
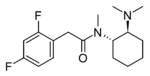
|
2,4-Difluoro-U-48800 | ||
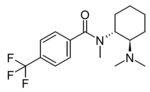
|
4-TFM-U-48520 (U-04) | 53720446 | 67579-38-8 |
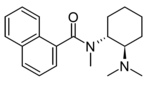
|
α-U10 | 165362154 | 2417942-61-9 |
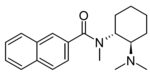
|
β-U10 | 54524276 | 67579-80-0 |
See also
References
- ↑ "Utopioids". Cayman Chemical News and Announcements. 12 July 2018. https://www.caymanchem.com/news/utopioids.
- ↑ "Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review". Neuropharmacology 134 (Pt A): 121–132. May 2018. doi:10.1016/j.neuropharm.2017.10.016. PMID 29042317. https://escholarship.org/uc/item/8xh0s7nf.
- ↑ "The search for the "next" euphoric non-fentanil novel synthetic opioids on the illicit drugs market: current status and horizon scanning". Forensic Toxicology 37 (1): 1–16. 2019. doi:10.1007/s11419-018-0454-5. PMID 30636980.
- ↑ "DARK Classics in Chemical Neuroscience: U-47700". ACS Chemical Neuroscience 11 (23): 3928–3936. December 2020. doi:10.1021/acschemneuro.0c00330. PMID 32639714.
- ↑ "U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market". Brain Sciences 10 (11): 895. November 2020. doi:10.3390/brainsci10110895. PMID 33238449.
- ↑ "Characterization of recent non-fentanyl synthetic opioids via three different in vitro µ-opioid receptor activation assays". Archives of Toxicology 96 (3): 877–897. March 2022. doi:10.1007/s00204-021-03207-9. PMID 35072756.
- ↑ "Investigation of the μ- and κ-opioid receptor activation by eight new synthetic opioids using the [35 S]-GTPγS assay: U-47700, isopropyl U-47700, U-49900, U-47931E, N-methyl U-47931E, U-51754, U-48520, and U-48800". Drug Testing and Analysis 14 (7): 1187–1199. July 2022. doi:10.1002/dta.3238. PMID 35142070.
- ↑ "Non-fentanyl opioids and related new psychoactive substances with no known legitimate uses.". International Narcotics Control Board. 28 January 2022. http://www.incb.org/documents/Global_Projects_OPIOIDS/INCB.GRIDS.OPIOIDS.NoFOs_list.pdf.
- ↑ "In vitro μ-opioid receptor activation potential of U10 and β-U10, positional isomers of the synthetic opioid naphthyl U-47700". Drug Testing and Analysis. July 2023. doi:10.1002/dta.3554. PMID 37482925.
 |

