Chemistry:Zeocin
| |
| Names | |
|---|---|
| Systematic IUPAC name
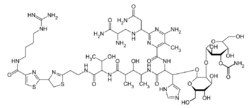
(24R,7S,10S,11S,12R,15S,16R,182R,183S,184S,185S,186S,192R,193S,194S,195R,196R)-15-{6-Amino-2-[(1S)-3-amino-1-{[(2S)-2,3-diamino-3-oxopropyl]amino}-3-oxopropyl]-5-methylpyrimidine-4-carboxamido}-14-({4-[(diaminomethylidene)amino]butyl}carbamoyl)-11,184,185,203,205-pentahydroxy-7-[(1R)-1-hydroxyethyl]-186,206-bis(hydroxymethyl)-16-(1H-imidazol-5-yl)-10,12-dimethyl-6,9,14-trioxo-24,25-dihydro-17,19-dioxa-5,8,13-triaza-1(2),2(4,2)-bis([1,3]thiazola)-18(2,3),20(2)-bis(oxana)icosaphan-204-yl carbamate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C55H86N20O21S2 | |
| Molar mass | 1427.53 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zeocin is a trade name for a formulation of phleomycin D1, a glycopeptide antibiotic and one of the phleomycins from Streptomyces verticillus belonging to the bleomycin family of antibiotics.[1] It is a broad-spectrum antibiotic that is effective against most aerobic organisms including bacteria, filamentous fungi, yeast, plant, and animal cells. It causes cell death by intercalating into DNA and inducing double stranded breaks of the DNA.[2][3]
Zeocin is a registered trademark belonging to InvivoGen. [4]
Properties
Zeocin is blue in colour due to the presence of copper ion Cu2+. This copper-chelated form is inactive. When Zeocin enters a cell, the Cu2+ is reduced to Cu+ and then removed. Subsequently, Zeocin becomes activated and can then bind and cleave DNA. However, the mechanism of action is not yet fully understood.[5]
Usage
Zeocin and other related chemicals in the bleomycin family of compounds are primarily used in molecular biology as an antibiotic, especially for the selection of eukaryotic cell lines when used in conjunction with vectors containing a selectable marker for Zeocin resistance. Zeocin is considerably cheaper than phleomycin, works better in minimal media, and is therefore often used preferentially in studies.[6]
Resistance to Zeocin is conferred by the product of the Sh ble gene first isolated from Streptoalloteichus hindustanus.[7] The Sh ble gene product binds the antibiotic in a one-to-one ratio so it can no longer cause cleavage of DNA. This resistance gene is used as a selectable marker in some cloning and expression vectors where Zeocin is used as the antibiotic for selection.[8][9]
Plasmids with Zeocin Resistance
pFUSE-Fc plasmid[citation needed]
pUNO1-Sh ble[10]
pSELECT-zeo[11]
pSELECT-GFPzeo[12]
References
- ↑ InvivoGen. "Technical Data Sheet for Zeocin". https://www.invivogen.com/sites/default/files/invivogen/products/files/zeocin_tds.pdf.
- ↑ "Copper-dependent cleavage of DNA by bleomycin". Biochemistry 26 (3): 931–42. 1987. doi:10.1021/bi00377a038. PMID 2436656.
- ↑ "Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response". Radiation and Environmental Biophysics 46 (4): 409–16. 2007. doi:10.1007/s00411-007-0123-2. PMID 17639449.
- ↑ "ZEOCIN Trademark of INVIVOGEN - Registration Number 6366745 - Serial Number 79285216 :: Justia Trademarks". https://trademarks.justia.com/792/85/zeocin-79285216.html.
- ↑ "Zeocin for selection of bleMX6 resistance in fission yeast". BioTechniques 51 (1): 57–60. 2011. doi:10.2144/000113706. PMID 21781055. http://www.biotechniques.com/multimedia/archive/00162/BTN_A_000113706_O_162844a.pdf.
- ↑ "Zeocin for selection of bleMX6 resistance in fission yeast". BioTechniques 51 (1): 57–60. 2011. doi:10.2144/000113706. PMID 21781055. http://www.biotechniques.com/multimedia/archive/00162/BTN_A_000113706_O_162844a.pdf.
- ↑ Gatignol, A., Durand, H. & Tiraby, G. (1988). "Bleomycin resistance conferred by a drug-binding protein". FEBS Lett 230 (1–2): 171–175. doi:10.1016/0014-5793(88)80665-3. PMID 2450783.
- ↑ "Baculovirus immediate-early promoter-mediated expression of the Zeocin resistance gene for use as a dominant selectable marker in dipteran and lepidopteran insect cell lines". Gene 188 (2): 183–90. 1997. doi:10.1016/s0378-1119(96)00756-1. PMID 9133590.
- ↑ "Phleomycin resistance as a dominant selectable marker in CHO cells". Somatic Cell and Molecular Genetics 14 (3): 243–52. 1988. doi:10.1007/bf01534585. PMID 2453083.
- ↑ InvivoGen (25 November 2016). "Zeocin Resistance plasmid". https://www.invivogen.com/puno-shble.
- ↑ InvivoGen (25 November 2016). "Zeocin Resistance plasmid". https://www.invivogen.com/pselect-zeo.
- ↑ InvivoGen (25 November 2016). "Zeocin Resistance plasmid". https://www.invivogen.com/pselect-gfpzeo.
External links
- "Zeocin". InvivoGen. 25 November 2016. https://www.invivogen.com/zeocin.
 |
