Chemistry:δ-Decalactone
From HandWiki
| |
| Names | |
|---|---|
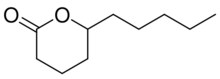
| Preferred IUPAC name
6-Pentyloxan-2-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H18O2 | |
| Molar mass | 170.252 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
δ-Decalactone (DDL) is a chemical compound, classified as a lactone, that naturally occurs in fruit[1] and milk products[2] in traces. It can be obtained from both chemical and biological sources.[3][4] Chemically, it is produced from Baeyer–Villiger oxidation of delfone.[5] From biomass, it can be produced via the hydrogenation of 6-amyl-α-pyrone.[6] DDL has applications in food,[7] polymer,[8] and agricultural[9] industries to formulate important products.
The S-enantiomer smells good. The R-enantiomer is the main component of the warning stench of the North American porcupine.[10]
See also
- γ-Decalactone
References
- ↑ Tamura, Hirotoshi; Appel, Markus; Richling, Elke; Schreier, Peter (2005). "Authenticity Assessment of γ- and δ-Decalactone from Prunus Fruits by Gas Chromatography Combustion/Pyrolysis Isotope Ratio Mass Spectrometry (GC-C/P-IRMS)". Journal of Agricultural and Food Chemistry 53 (13): 5397–5401. doi:10.1021/jf0503964. PMID 15969525.
- ↑ Karagül-Yüceer, Yonca; Drake, Maryanne; Cadwallader, Keith R. (2001). "Aroma-Active Components of Nonfat Dry Milk". Journal of Agricultural and Food Chemistry 49 (6): 2948–2953. doi:10.1021/jf0009854. PMID 11409991.
- ↑ Corma, Avelino; Iborra, Sara; Mifsud, María; Renz, Michael; Susarte, Manuel (2004). "A New Environmentally Benign Catalytic Process for the Asymmetric Synthesis of Lactones: Synthesis of the Flavouringδ-Decalactone Molecule". Advanced Synthesis & Catalysis 346 (23): 257–262. doi:10.1002/adsc.200303234.
- ↑ Alam, Md. Imteyaz; Khan, Tuhin S.; Haider, M. Ali (2019). "Alternate Biobased Route to Produce δ-Decalactone: Elucidating the Role of Solvent and Hydrogen Evolution in Catalytic Transfer Hydrogenation". ACS Sustainable Chemistry & Engineering 7 (3): 2894–2898. doi:10.1021/acssuschemeng.8b05014.
- ↑ Corma, Avelino; Iborra, Sara; Mifsud, María; Renz, Michael; Susarte, Manuel (2004). "A New Environmentally Benign Catalytic Process for the Asymmetric Synthesis of Lactones: Synthesis of the Flavouring δ-Decalactone Molecule". Advanced Synthesis & Catalysis 346 (23): 257–262. doi:10.1002/adsc.200303234.
- ↑ Alam, Md. Imteyaz; Khan, Tuhin S.; Haider, M. Ali (2019). "Alternate Biobased Route to Produce δ-Decalactone: Elucidating the Role of Solvent and Hydrogen Evolution in Catalytic Transfer Hydrogenation". ACS Sustainable Chemistry & Engineering 7 (3): 2894–2898. doi:10.1021/acssuschemeng.8b05014.
- ↑ The forty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives; WHO food additive series 40; WHO: Geneva, 1998
- ↑ Martello, Mark T.; Burns, Adam; Hillmyer, Marc (2012). "Bulk Ring-Opening Transesterification Polymerization of the Renewable δ-Decalactone Using an Organocatalyst". ACS Macro Letters 1: 131–135. doi:10.1021/mz200006s.
- ↑ Menger, D. J.; Van Loon, J. J. A.; Takken, W. (2014). "Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host". Medical and Veterinary Entomology 28 (4): 407–413. doi:10.1111/mve.12061. PMID 24797537.
- ↑ Li, Guang; Roze, Uldis; Locke, David C. (December 1997). "Warning Odor of the North American Porcupine(Erethizon dorsatum)". Journal of Chemical Ecology 23 (12): 2737–2754. doi:10.1023/A:1022511026529. Bibcode: 1997JSP....23.2737L.

