Medicine:Aortic valve repair
| Aortic valve repair | |
|---|---|
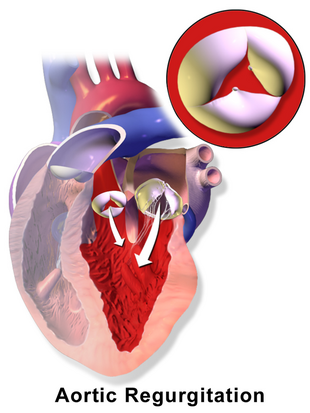 In aortic regurgitation the cusps do not close completely during the filling phase of the heart (diastole), there is backflow of blood into the left ventricle. | |
| Other names | Aortic valve reconstruction |
| Specialty | Cardiology |
| ICD-9-CM | 35.9 |
Aortic valve repair or aortic valve reconstruction is the reconstruction of both form and function of a dysfunctional aortic valve. Most frequently it is used for the treatment of aortic regurgitation.[1] It can also become necessary for the treatment of aortic aneurysm, less frequently for congenital aortic stenosis.
Background
An aortic valve repair will realistically be possible in the absence of calcification or shrinking (retraction) of the aortic valve. Thus, congenital aortic stenosis may be treated by aortic valve repair.[2] In acquired aortic stenosis valve replacement will be the only realistic option. In most instances, aortic valve repair will be performed for aortic regurgitation (insufficiency).[3] Aortic valve repair may also be performed in the treatment of aortic aneurysm or aortic dissection if either aneurysm or dissection involves the aorta close to the valve.[4]
Indications for aortic valve repair:
- Absence of relevant calcification and
- Congenital and severe aortic stenosis with symptoms or decreased left ventricular function
- Severe aortic regurgitation and symptoms, or leftventricular enlargement (>65 to 70 mm), or decreased left ventricular function (EF < 50%)
- Ascending aortic aneurysm > 55mm
- Ascending aortic aneurysm > 50mm and risk factors (e.g. high blood pressure)
- Ascending aortic aneurysm > 50mm and connective tissue disease
- Ascending aortic aneurysm > 50mm and risk factors and connective tissue disease[5]
Repair versus replacement
The goal of the operation is the improvement of life expectancy and treatment of heart failure as the consequence of dysfunction of the aortic valve. The goal may also be to avert complications of the aorta (rupture or dissection) in the treatment of aneurysm. Repair is a more recent alternative to replacement; in many instances replacement will be the only realistic option because of severe destruction of the valve.[6]
While replacement of the aortic valve is a safe and reproducible procedure it may still be associated with the long-term occurrence of so-called valve-related complications. The probability of these complications depends on the age of the patient and the type of operation.[7] Typical complications are blood clot formation on the valve or dislodgment of thrombus (embolism); bleeding complications are commonly a consequence of "blood-thinning" medication needed to prevent clots (anticoagulation). Biologic/tissue replacement valves have a tendency to degenerate, and there is also an increased risk of infections of valve prosthesis (prosthetic valve endocarditis).
Compared to the results of valve replacement there will be a minimal tendency towards clot formation after aortic valve repair, and anticoagulation is commonly not necessary, thus minimizing the possibility of bleeding complications. The likelihood of infection of the repaired aortic valve is much lower compared to what is seen after aortic valve replacement.[8] A repair procedure may not last forever, but in many instances the durability of an aortic valve repair will markedly exceed that of a biological prosthesis.[6]
Surgical technique
The details of the aortic valve repair procedure depend on the possibility of congenital malformation of the valve, the type and degree of secondary deformation, and the existence of an aortic aneurysm. The goal of the procedure is the restoration of a normal form of the aortic valve, which will then lead to near-normal function and good durability of the repair. A transesophageal echocardiogram during the operation and prior to the repair will be important to define the exact deformation of the aortic valve and thus the mechanism of regurgitation.[citation needed]
In order to best accommodate the complex geometry of the aortic valve, these procedures are generally performed through open-heart surgery. Minimally invasive procedures limit the ability to precisely judge the form of the aortic valve and will lead to a higher uncertainty regarding function and durability of aortic valve repair. As for aortic valve replacement, the heart-lung machine is usually connected to the patient via aorta and right atrium. The heart is arrested through cardioplegia, and the form of the aortic valve is carefully analyzed. Currently documented predicted values for certain aspects of the form of the aortic valve.[9] are available. Using these parameters and a good transesophageal echocardiogram the precise mechanism of regurgitation can be determined in most cases.[10]
Aortic valve stenosis
Congenital aortic valve stenosis can be treated by aortic valve repair if there is no relevant calcification.[2] In this scenario the aortic valve will almost always be unicuspid and the valve configuration must be altered as part of the procedure in order to improve opening of the valve. Because of the unicuspid form of the valve the repair concept will be similar to that of the regurgitant unicuspid valve.
The traditional treatment of congenital aortic stenosis is balloon valvuloplasty or surgical commissurotomy. Both approaches will frequently not eliminate the narrowing of the valve; in addition, they will lead to a variable degree of aortic valve regurgitation which places an additional burden on the heart. In both interventions some of the valve tissue is opened; the peculiar form aspects of unicuspid aortic valves are not taken into consideration.[11] The repair approach differs from commissurotomy mainly in that not only valve tissue is divided to improve opening, but also at least an additional commissure (suspension point of the valve) is created for the aortic valve. Thus, a bicuspid valve is created which results in near-normal function of the aortic valve.
The most reproducible concept is the creation of a bicuspid aortic valve with two normal commissures and two cusps. Tissue of the aortic valve is removed or detached from the aorta in places where it is clearly abnormal. The location of a second commissure of normal height is determined; using a patch or the original cusp tissue the cusps are then sutured to the aortic wall in order to create cusps of sufficient tissue and adequate form reaching the new commissure.
Aortic regurgitation
Tricuspid aortic valve
In tricuspid aortic valves the anatomy is principally normal; if there is an aneurysm of the ascending aorta the principles of aortic aneurysm will have to be applied. Without aneurysm, the cause of regurgitation is frequently stretching of one or two of the valve components (cusps). Such stretching can be combined with the presence of congenital tissue fenestrations. Additionally, enlargement of the aortic annulus can contribute to valve dysfunction. Shrinkage of the cusps is less frequent in industrial countries; this is currently not well treatable by repair.[citation needed]
In surgical treatment, the extent of cusp stretching is exactly determined and then corrected by sutures. Enlargement of the annulus requires its size reduction and stabilization by an annuloplasty. In the case of annular dilatation, the annulus has to be reduced; currently, the largest experience exists with a strong suture that is placed around the annulus and tied to the desired size. Stretching is corrected by plicating sutures to the point that all cusps have a normal configuration. At the end of the operation, the cusp margins should be at an identical height.[citation needed]
Bicuspid aortic valve
File:Aortenklappe präoperativ.tif
In bicuspid aortic valve anatomy, there is congenital fusion of two cusps. This fused cusp is exposed to higher than normal stress and will stretch over time as a consequence. This results in aortic valve regurgitation. Annular enlargement is very frequent in this context, and it increases the tendency to leak. As a result of long-standing dysfunction also the normal cusp may undergo deformation and stretch. In half of the affected individuals there is also an aneurysm of the ascending aorta which has to be treated appropriately.[citation needed]
File:Aortenklappe postoperativ.tif Since the bicuspid anatomy commonly has an almost normal valve function (unless deformed) it is left bicuspid; the repair procedure simply corrects the secondary deformations that led to regurgitation. Similar to tricuspid aortic valves, the cusps must be measured to rule out shrinkage. The annulus is commonly enlarged, it must be reduced and stabilized by an annuloplasty.[12] Tissue redundancy through stretching is corrected by sutures.
Unicuspid aortic valve
The unicuspid aortic valve may not only result in relevant stenosis (narrowing), it may also primarily lead to regurgitation. In a proportion of the affected individuals, an aneurysm of the ascending aorta may be present which may need treatment as well. The repair procedure will change the configuration of the valve by creating at least one additional commissure. Commonly the unicuspid valve is changed into a bicuspid configuration; the resulting valve function will be close to normal. Cusp tissue is resected where it is grossly abnormal. Using patch tissue, the cusps are enlarged so they reach the second (new) commissure. If the annulus is enlarged it must be reduced and stabilized.[12]
Quadricuspid aortic valve
Aortic regurgitation in a quadricuspid valve is commonly caused by the additional (4th) commissure, which holds back cusp tissue and keeps it from closing adequately. Currently, the most reliable concept for repair of a quadricuspid valve seems to be its conversion into a tricuspid valve.[13] In some cases a bicuspid configuration may be appropriate. In order to achieve this cusp, tissue is detached from the aorta and the valve is then brought into adequate form.
Aneurysm of the ascending aorta
The enlargement of the ascending aorta may lead to aortic valve regurgitation because the outward tension on the cusps prevents their adequate closure. Regurgitation may also (in part) be due to congenital malformation of the aortic valve or concomitant stretching of a tricuspid aortic valve. Life expectancy may be limited by severe aortic regurgitation. The aneurysm of the ascending aorta may also become so large that it can develop rupture or dissection as life-threatening complications.[citation needed]
The operation must address the aneurysm by replacing the enlarged part of the aorta. Since the aortic valve is very sensitive in its form and function to any changes of the aortic dimensions, the operation will in most cases also have to address the valve, i.e. apply the principles of aortic valve repair. This principle applies to tricuspid valves as well as bicuspid or unicuspid aortic valves.[14][15]
The goal of the operation is to eliminate the aneurysm and to preserve or repair the aortic valve. The operation may include replacement of the aortic root. Replacement of the root is usually not necessary if its diameter is less than 40 to 45 mm. In those instances replacement of the ascending aorta is sufficient. If root diameter exceeds 45 mm it will have to be replaced in many instances. There are mainly 2 operative techniques currently used,[16][17] and both lead to similar results.[18][19] With both techniques the aortic valve must be carefully assessed after replacement of the root; repair of any aortic valve abnormalities is necessary in order to achieve good and durable valve function.[19][20]
- Operative details
There are two options: tubular ascending aortic replacement or replacement of the aortic root.
Tubular ascending aortic replacement
The aorta is divided above the aortic valve and root. The avascular graft is then sutured to the aortic root. The form of the aortic valve may have been changed by this maneuver, it thus has to be carefully checked. Often stretching of a cusp becomes apparent at that point, and this will have to correct by sutures (see 3.3.1, 3.3.2).
Replacement of the aortic root
After the heart has been arrested, the enlarged aorta is removed close to the insertion line of the aortic valve cusps. The origins of the coronary arteries must be detached from the aorta. For the procedure, according to Magdi Yacoub[16] a graft is tailored to create 3 tongues that replace the aneurysmatic aortic wall in the root. The graft is then sutured to the cusp insertion lines. Some surgeons combine this procedure with an annuloplasty.[12][21] For the procedure according to Tirone David,[17] the aortic valve is mobilized even further from the surrounding tissues. The avascular graft is then positioned around the valve, and the valve is fixed inside the graft with sutures.
With both techniques, the form of the aortic valve must be carefully assessed after completed root replacement.[22] In most instances some cusp stretching will be found which would result in prolapse and relevant regurgitation afterward if uncorrected. Thus an aortic valve repair procedure will frequently be necessary according to the principles of tricuspid or bicuspid aortic valve repair.
Postoperative treatment
Contrary to valve replacement with mechanical prostheses inhibition of the blood clotting system (anticoagulation) is not necessary after aortic valve repair. Blood-thinning may only be necessary if atrial fibrillation occurs or persists in order to prevent blood clot formation in the left atrium.[citation needed]
Following aortic valve replacement, prophylactic administration of antibiotics is recommended for interventions involving mouth and throat (e.g. dental surgery).[4] It is unclear whether this is also necessary after aortic valve repair.
History
First attempts at aortic valve repair were undertaken even before heart valve prostheses were developed. In 1912 the French surgeon Theodore Tuffier widened a stenotic (narrowed) aortic valve. The colleagues of Dwight Harken reported in 1958 on their experience with aortic valve repair for aortic regurgitation by narrowing the annulus of the aortic valve.[23] In those times, both surgeons and cardiologists had minimal information on the exact nature and severity of dysfunction of the aortic valve. This changed with the development of echocardiography by Inge Edler and Carl Hellmuth Hertz in the early 1950s. Nonetheless, the development of heart valve prostheses made replacement the standard approach because of its reproducibility. The first ball-cage valve was implanted in 1961 by the American surgeons Albert Starr and Lowell Edwards,[24] and in the next decades many mechanical and biological prostheses were developed and used. The positive results with the repair of the mitral valve stimulated surgeons in the 1980s and 1990s to develop surgical techniques that could be applied for the different causes of aortic regurgitation. Stepwise improvements were introduced in the subsequent years; today many regurgitant aortic valves can be treated by repair.
References
- ↑ "Aortic Regurgitation". https://www.lecturio.com/concepts/aortic-regurgitation/.
- ↑ 2.0 2.1 Schäfers HJ, et al. Bicuspidization of the unicuspid aortic valve: a new reconstructive approach. Ann Thorac Surg. 2008 Jun;85(6):2012–2018.
- ↑ Aicher D, Schäfers HJ. Aortic valve repair – current status, indications, and outcomes. Semin Thorac Cardiovasc Surg. 2012;24(3):195–201
- ↑ 4.0 4.1 Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012 Oct;33(19):2451–2496
- ↑ Ahmed, T; Puckett, Y (2021). Aortic Valve Repair. PMID 32644365. https://www.ncbi.nlm.nih.gov/books/NBK558939/. Retrieved 29 June 2021.
- ↑ 6.0 6.1 Regeer, M; Versteegh, M; Klautz, R; Stijnen, T; Schalij, M; Bax, J; Marsan, N; Delgado, V (17 October 2014). "Aortic valve repair versus replacement for aortic regurgitation: effects on left ventricular remodeling". Cardiothoracic Surgery Journals 30 (1): 13–19. doi:10.1111/jocs.12457. PMID 25327584. https://pubmed.ncbi.nlm.nih.gov/25327584/. Retrieved 29 June 2021.
- ↑ Hammermeister K, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158.
- ↑ Aicher D, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg. 2010;37:127–132
- ↑ Hans-Joachim Schäfers: Current treatment of aortic regurgitation. UNI-MED Science, Bremen, London, Boston 2013, ISBN 978-3837414066
- ↑ Langer F, et al. Aortic valve repair using a differentiated surgical strategy. Circulation. 2004;110:II67–73
- ↑ Anderson RH. Understanding the structure of the unicuspid and unicommissural aortic valve. J Heart Valve Dis. 2003 Nov;12(6):670–673
- ↑ 12.0 12.1 12.2 Aicher D, Schneider U, Schmied W, Kunihara T, Tochii M, Schäfers HJ. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg. 2013 Mar;145(3 Suppl):pp. 30–34
- ↑ Schmidt KI, et al. Tricuspidization of the quadricuspid aortic valve. Ann Thorac Surg. 2008 Mar;85(3):1087–10879
- ↑ "Remodeling of the aortic root and reconstruction of the bicuspid aortic valve". Ann Thorac Surg 70 (2): 542–546. 2000. doi:10.1016/s0003-4975(00)01457-0. PMID 10969677.
- ↑ "Root remodeling and aortic valve repair for unicuspid aortic valve". Ann Thorac Surg 98 (3): 823–829. Sep 2014. doi:10.1016/j.athoracsur.2014.05.024. PMID 25085562.
- ↑ 16.0 16.1 "Remodeling of the aortic valve annulus". J Thorac Cardiovasc Surg 105 (3): 435–438. 1993. doi:10.1016/S0022-5223(19)34225-4. PMID 8445922.
- ↑ 17.0 17.1 "An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta". J Thorac Cardiovasc Surg 103 (4): 617–621. Apr 1992. doi:10.1016/S0022-5223(19)34942-6. PMID 1532219.
- ↑ "Reexamining remodeling". J Thorac Cardiovasc Surg 149 (2 Suppl): S30–36. Feb 2015. doi:10.1016/j.jtcvs.2014.09.048. PMID 25439784.
- ↑ 19.0 19.1 "Current readings: Aortic valve-sparing operations". Semin Thorac Cardiovasc Surg 26 (3): 231–238. Autumn 2014. doi:10.1053/j.semtcvs.2014.10.002. PMID 25527017.
- ↑ "Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair". J Thorac Cardiovasc Surg 143 (6): 1389–1395. Jun 2012. doi:10.1016/j.jtcvs.2011.07.036. PMID 21855091.
- ↑ "An aortic ring: From physiologic reconstruction of the root to a standardized approach for aortic valve repair". J Thorac Cardiovasc Surg 140 (6): S28–35. 2010. doi:10.1016/j.jtcvs.2010.08.004. PMID 21092793.
- ↑ "A new approach to the assessment of aortic cusp geometry". J Thorac Cardiovasc Surg 132 (2): 436–438. 2006. doi:10.1016/j.jtcvs.2006.04.032. PMID 16872982.
- ↑ Taylor WJ, et al. The surgical correction of aortic insufficiency by circumcision. J Thorac Cardiovasc Surg. 1958;35:192–231.
- ↑ L. Wi Stephenson: History of Cardiac Surgery. In: L. H. Cohn, L. H. Edmunds Jr. (Hrsg.): Cardiac Surgery in the Adult. McGraw-Hill, New York 2003, S. 3–29.
Further reading
- Hans-Joachim Schäfers: Current treatment of aortic regurgitation. UNI-MED Science, Bremen, London, Boston 2013, ISBN 978-3837414066.
- Hans-Joachim Schäfers: Klinische Grundlagen der Herz- und Thoraxchirurgie. 1. Auflage. ABW Wissenschaftsverlagsgesellschaft, Berlin 2003.
External links
 |
